Below you will find a collection of all FAQs posted across the College’s website. Please use the category list to locate the topic you are looking for.
Administering Injections
No. Pharmacists should retain their own documentation as evidence that they have completed theoretical and practical training, as well as an assessment of their competency, prior to engaging in this area of practice. This information can be included in their Learning Portfolio and provided if requested (by a patient, employer, the College, etc.). This information will not appear on the public register, Find a Pharmacy or Pharmacy Professional and the Glossary of Terms has been updated to explain that “Trained to administer injections” means the pharmacy professional has declared completion of a College-approved injection training course to administer subcutaneous (SC) and intramuscular (IM) injections. SC injections are administered in the fat layer underneath the skin (for example, insulin). IM injections are delivered into the muscle (for example, a flu shot). The substances a pharmacist or pharmacy technician are permitted to administer by injection are listed in Schedule 1 and Schedule 3 (Vaccines) of Ontario Regulation 256/24.
The College’s Administering a Substance by Injection Guideline does not mandate specific requirements for injection recertification or training updates.
After registering your injection training, a self-declaration is made annually at registration renewal indicating that you have completed a College-approved training course and will maintain valid First Aid & CPR certification.
The Standards of Practice and Code of Ethics expect pharmacy professionals to recognize and work within the limits of their competence and to only practice when they have the appropriate knowledge and skill, as well as the physical, emotional and mental capacity, to do so safely.
Pharmacists and pharmacy technicians are committed to continuous lifelong learning and professional improvement. It is your responsibility to self-assess your ability to administer injections prior to engaging in this practice. Should you wish to pursue additional training, there are continuing education opportunities available on the Continuing Education for Pharmacists and Pharmacy Technicians page.
Yes. Your supervisor must have completed an OCP-approved injection training course, ensured their First Aid and CPR certification is up to date, and registered their training with the College in order for you to administer an injection under their supervision. For further guidance on the role of your supervisor, see OCP’s Supervision of Pharmacy Personnel Policy.
Please refer to the e-Learning module on Understanding Drug Schedules and Injection Administration.
AIMS Program
Only aggregate and de-identified data collected through the program will be reported publicly. Data reports from the years 2020 to 2024 are available.
Starting in 2027, data reported by Ontario pharmacies in their incident management platforms will be uploaded to ISMP’s National Incident Data Repository for Community Pharmacies. This data will be used nationally to help identify widespread risks and trends and enable more effective coordinated interventions. It will also support consistency in safety practices, policy development, and shared learning across jurisdictions, ultimately improving patient safety across the country.
As of January 1, 2027, registered pharmacy staff (pharmacists and pharmacy technicians) must have a unique login at their primary place of practice. Occasional staff, such as relief pharmacists and pharmacy technicians, would not be required to have individual logins. Unregulated staff, such as pharmacy assistants, are not required to have access; however, access may be granted at the discretion of the Designated Manager when appropriate.
No information that could identify a patient is submitted from a pharmacy’s incident management platform to the National Incident Data Repository (NIDR). Only the patient’s age range and gender will be shared. It is the responsibility of the pharmacy to ensure that there is no identifiable information provided in any free text fields.
As health information custodians, pharmacies are accountable for taking reasonable steps to protect personal health information and keep it secure.
No. The College only has access to aggregate data provided through the National Incident Data Repository. However, pharmacy staff may be asked to provide proof (after January 1, 2027) that they have an incident management platform in place that meets the College’s criteria and that it is being used in alignment with the AIMS Program requirements.
To fulfill the requirements of the AIMS Program, pharmacies must have access to an incident management platform that supports continuous quality improvement by January 1, 2027. It must meet the Incident Management Platform Criteria set out by the College.
If a potential error is caught outside of the established processes and procedures at the pharmacy but before the prescription reaches the patient, it should be recorded as a near miss. Established processes and procedures could include the technical and therapeutic signoffs and/or any other regular process in place to catch errors such as input or DIN errors.
Regardless of when a near miss is caught, if it is noted that similar errors are re-occurring on a frequent basis, this may indicate that the processes and procedures implemented into the workflow are not effective and should be reviewed.
The extent to which near misses are recorded will be a professional judgment decision of the Designated Manager in consideration of the nature of the near miss, its implication for patient safety, and the extent to which it is recurring.
If a medication incident occurs, it is the College’s expectation that the pharmacy staff act promptly to provide appropriate support for the patient. They must record the incident and activate the quality improvement process. This includes documenting what happened, analyzing the incident to determine contributing factors, working to identify how it can be prevented from recurring, and taking the necessary steps to accomplish that goal by applying and sharing quality improvement strategies with their teams.
The same process must also be followed for near misses, which provide valuable insight into areas of risk, and may indicate where systems can be improved to prevent harm.
All registered pharmacy professionals must meet the requirements of the supplemental Standard of Practice.
All community pharmacies are required to meet the requirements of the AIMS Program.
In accordance with the Standards of Operation, hospitals must support pharmacy professionals in meeting the requirements of the supplemental Standard of Practice by reporting incidents involving medications to the incident management system.
The goal of the Assurance and Improvement in Medication Safety (AIMS) Program is to reduce the risk of patient harm caused by medication incidents in, or involving, Ontario pharmacies.
The program sets out a mandatory standard for medication safety to enable all pharmacies to use a consistent approach to reporting, documenting, analyzing and sharing learnings related to medication incidents. It supports a safety culture that enables continuous quality improvement at an individual pharmacy level and system wide.
Starting in 2027, information collected through the incident management platform and provided to the National Incident Data Repository for Community Pharmacies (NIDR) will also help to identify trends and develop recommendations for pharmacy professionals across the country.
Annual Fees
Registrants may pay their annual fees when it is time to renew their certificates of registration, usually starting in mid-January of each year through to mid-March. Please watch for specific details and deadlines communicated before and during each annual renewal period.
Registrants can pay their fees in several ways, including online through the secure registrant portal available on OCP’s website. Specific payment options will be confirmed by OCP before and during each renewal period.
Your fees contribute directly to the effective regulation of pharmacy in the public interest.
The College is required to do several important things to fulfill our mandate, including meeting the “objects” spelled out in the Regulated Health Professions Act, which applies to all health professional regulators in Ontario. Our role is to ensure those who are registered to practice in Ontario are qualified and competent and are providing care that meets expected practice and ethical standards so that the public can be assured that the services they receive from their pharmacy professional are safe, ethical and focused on their interests.
The College uses annual fees to deliver on this mandate and Board-defined priorities. In other words, your fees fund the College’s activities and the regulatory programs we deliver. For example, we:
Manage registration and quality assurance programs.
We do this by:
- Registering qualified and competent professionals to practice in Ontario, assuring the public that they can expect quality, safe and ethical care from their pharmacy professionals. This includes processing first-time registration applications and related activities, the annual renewal of certificates of registration and administration of the Registration Committee.
- Overseeing the quality and safety of the practice of Ontario’s approximately 25,000 pharmacists and pharmacy technicians. This includes conducting coaching-focused practice assessments and supporting practice improvement and competence, developing policies, providing guidance, establishing an Equity, Diversity and Inclusion strategy and administration of the Quality Assurance Committee.
- Overseeing the quality and safety of Ontario’s 4,900+ community and 230+ hospital pharmacies. This includes conducting operational assessments, applications and renewals and administration of the Accreditation and Drug Preparation Premises Committees.
Promote quality and safe pharmacy practice.
We do this by:
- Ensuring standards and policies are continually reviewed and updated to ensure that they remain relevant and reflect current practice and the evolving needs of Ontario’s health system.
- Supporting the well-being of pharmacy professionals through its voluntary Ontario Pharmacy Health Program which encourages and supports registrants to seek treatment for substance use or other mental health disorders that may affect their ability to practice safely.
- Updating and making regulations to ensure the ongoing safety of the public as pharmacy professionals expand their scopes of practice.
- Providing relevant and timely guidance, information and other resources to promote safe, quality pharmacy practice.
Respond to concerns about registrant conduct.
We do this by:
- Investigating complaints and concerns about the practice or conduct of pharmacy professionals. This includes responding to complaints and reports, conducting thorough and impartial investigations, promoting remediation and practice improvement and the administration of the Inquiries, Complaints and Reports Committee.
- Holding professionals accountable for conduct that falls below expectations of the profession. This includes legal and other costs associated with preparing for, conducting and concluding proceedings of the Discipline Committee and Fitness to Practise Committee.
Ensure we have the resources and infrastructure necessary to fulfill our mandate effectively and efficiently.
We do this by:
- Establishing and maintaining the technology, facility and information infrastructure to carry out our regulatory duties. This includes equipping and maintaining technology that supports a remote workforce and having systems that safely manage and secure our data.
- Ensuring that we are accountable and responsible stewards of our budget and the fees we collect in the administration of our mandate.
- Implementing good governance practices and supporting the administration of Board and Committee activities across the College.
As the work of the College is set out in legislation, your fees are essential to our duty to regulate the profession in the public interest. Like all self-regulated healthcare professionals in Ontario, pharmacists and pharmacy technicians must be registered with their licensing body to practice. This means that as a pharmacist or pharmacy technician, you must register (and renew) with the Ontario College of Pharmacists, and pay the required fee, to legally practice in the province.
Annual Registrant Renewal
This notification means that you have been selected to complete the Knowledge Assessment as part of the College’s mandatory Quality Assurance (QA) Program.
The Knowledge Assessment is an open-book, online assessment that evaluates a Part A pharmacist’s ability to apply clinical knowledge as well as current legislation, ethics and scope of practice to patient care scenarios.
Selection for the Knowledge Assessment is conducted through a random process and participation is mandatory for Part A pharmacists who are selected.
For more information, including FAQs, please visit the Knowledge Assessment webpage.
If you received a pop-up notification that you’ve been selected for the Knowledge Assessment but haven’t received an email notification confirming your selection, please contact [email protected].
Registrants must demonstrate sufficient English or French language proficiency throughout their registration with the College. Language proficiency requirements help ensure pharmacy professionals can effectively communicate with patients and other healthcare professionals to support safe and quality patient care throughout their pharmacy careers.
All pharmacists and pharmacy technicians (both Part A and Part B) must complete the “Declaration of Language Proficiency” as part of their annual renewal process. This helps ensure language proficiency requirements are met.
The College accepts credit card, cheque or money order. Please note the College’s portal does not accept Interac debit payment.
If you are a Part A registrant who will continue to work in the pharmacy profession but not provide patient care (as defined on the Part A & B Register page), and you wish to be able to use the protected title of “pharmacist” or “pharmacy technician”, you have the option to move to Part B of the Register. As a Part B registrant, you are required to complete the annual renewal but your renewal fee is lower and you do not need to have personal liability insurance. If you decide to return to providing patient care at a later date (no time limit), you will have to undergo the process to move from Part B to Part A. This option should be considered for a registrant who is changing to a non-patient care role.
If you are a pharmacist or pharmacy technician in good standing who will not be working in the profession in the near future but intend to return to practice within three years, you have the option to resign from the Register and then reinstate. While you are resigned from the Register, you will not be required to pay the annual renewal fee or maintain personal professional liability insurance. Please note that once resigned, you will not be able to use the protected title of “pharmacist” or “pharmacy technician” or provide patient care. This option is appropriate to consider for a practitioner who is taking a leave from practising as a pharmacist or pharmacy technician for less than three years (e.g., parental or personal leave, sabbatical, temporary relocation outside of Ontario, working outside the profession of pharmacy).
You are eligible to apply for reinstatement within three years of the date of your resignation. When seeking reinstatement, former registrants will be reinstated into Part B of the Register. Registrants may be reinstated to Part A of the Register if certain conditions are met.
After three years from the date of your resignation from the Register, you will not be eligible for reinstatement. If you wish to return to the profession, you will be required to re-apply as a new applicant. Please review the information about reinstatement after three years from the date of resignation on the Resigning & Reinstating web page. This option is appropriate for a practitioner who is relocating outside of Ontario for three years or longer, changing careers or retiring.
For any questions about the annual renewal process, please contact the Registrant Applications and Renewals team at [email protected] or 416-962-4861 / 1-800-220-1921 ext. 3400.
Revenue from annual fees is necessary for the College to meet our legislated obligations and regulate the profession in the public interest. College by-laws approved by the Board of Directors in 2020 set an annual increase in fees to be tied to the percentage increase, if any, in the Consumer Price Index as of September 30th of each year. The intention is to reduce the need for large increases in fees in any single year by keeping up with inflation, which impacts everyone and contributes to higher costs for all organizations, including the College.
As part of annual renewal, the College is inviting registrants to share their pronoun information to help the College use inclusive and accurate language during interactions with registrants. Pronouns refer to how you would like to be referred to in the third person and could include she/her, he/his or they/them. Sharing pronoun information with the College is optional and will only be used during staff-registrant interactions. The College’s commitment to safeguarding registrants’ privacy is reflected in our Privacy Policy.
Sharing your pronouns with the College is optional. If you do choose to share your pronouns, this information will only be used to support inclusive and accurate language during your interactions with College staff.
Registrants should set aside 10-15 minutes to complete their annual renewal. If there are changes to your personal and workplace information, you will be asked to update it. Registrants will also be asked to respond to declarations. These should be read carefully before responding.
All pharmacists and pharmacy technicians must complete the annual renewal process.
Interns, intern technicians and registrants holding an Emergency Assignment (EA) certificate of registration (when available), do not complete the annual renewal process.
As of Right
No. Those practicing under As of Right rules can only practice for a maximum of one six-month period. The six month period cannot be extended or used on more than one occasion. If College registration requirements are not met within the six-month window, those practicing under As of Right rules must immediately stop practicing in Ontario. Those practicing under As of Right rules must also immediately stop working in Ontario if they do not continue to meet all the eligibility criteria, or their College registration application is rejected
No. There are no practice setting restrictions for eligible applicants under the As of Right rules.
No. Until pharmacists or pharmacy technicians are registered with the College, they will not have a public profile on our Find a Pharmacy / Professional tool.
Six months. Pharmacists and pharmacy technicians already licensed in another Canadian province or territory who meet the eligibility criteria can practice for up to six months while completing their pharmacist registration requirements or pharmacy technician registration requirements with the College.
Board of Directors
Board meetings are generally held four times a year at the College’s office in Toronto. These public meetings are held in March, June, September and December, and are usually one or one-and-a-half days in duration.
The Board comprises elected pharmacists and pharmacy technicians, members of the public who are appointed by the provincial government, and academic appointees. Together, they share a duty to serve and protect the public interest.
Every Registrant who holds a valid Certificate of Registration as a pharmacist or a pharmacy technician, who practises or resides in Ontario, and who is not in default of payment of the annual fee, is entitled to vote in an election of Directors.
The application form can be found on the College’s Recruitment Page.
No. However, Board Directors are remunerated by an honorarium for their time spent participating in College activity. Elected Directors will be compensated in accordance with the College’s remuneration policy.
The College’s competency-based Board has put added emphasis on equity, diversity and inclusion, and we are focused on attracting qualified candidates with competence in financial oversight who are from diverse populations, marginalized groups or individuals with disabilities, or those who have experience working with these groups.
No. While the skills on the Director Profile must be present on the Board, they are not all required for every Board Director. The intent is to ensure the Board comprises a collection of individuals who bring different strengths to the table. Having any of the experience or core competencies identified in the Director Profile is an asset, but they are not all required for applying.
The total time commitment will vary depending on the level of involvement on committees and interested applicants should be prepared to serve between 15 and 30 days per year. Elected Board Directors will be appointed to serve on the Discipline Committee as required by the Regulated Health Professions Act (RHPA) and may also be considered for appointment to other committees of the Board.
At a high level, the Board approves policy, sets annual fees, sets the vision and strategic direction for the College, chooses the Registrar and CEO, and provides oversight for the College to ensure operations comply with Board direction. The Board’s primary role is to ensure that the interests of the public are protected.
If you have questions, you may contact the College at [email protected].
External governance consultants will conduct a short-listing for submission to the Screening Committee which would select the candidates qualified to seek election to the Board of Directors.
The Director Profile states the eligibility requirement for standing for election. It provides that a Registrant who holds a valid Certificate of Registration and lives and/or works in Ontario is eligible to seek to be a candidate for election to the Board if they meet the following eligibilities:
- Registrant is not in default of payment of any fees prescribed in the by-laws.
- Registrant is not the subject of any disciplinary or incapacity proceeding.
- Registrant has not been found to have committed an act of professional misconduct or to be incompetent by a panel of the Discipline Committee.
- Registrant is not a registered pharmacy student or intern.
- Registrant’s Certificate of Registration is not subject to a term, condition, or limitation other than one prescribed by regulation.
- Registrant is not and has not, within the three (3) years immediately preceding the election, been an employee, officer, or director of a Professional Advocacy Association.
- Registrant has not been disqualified from serving on the Board or a Committee within the six (6) years immediately preceding the election.
- Where the Registrant has served two (2) consecutive, three (3) year terms for a total of six (6) years, it has been at least three (3) years since they were a Director. (Some exceptions apply*)
*Registrants elected prior to 2020 who have served continuously since that time are eligible to complete three (3) consecutive, three (3) year terms for a total of nine (9) years. - Registrant is not an adverse party in litigation against the College, the Board, a Committee or any of the College’s officers, employees or agents.
- Registrant commits to devoting sufficient time in their schedule to participating in all required Board and Committee activities.
- Registrant has not, in the opinion of the Screening Committee, engaged in conduct unbecoming a Director.
- Registrant is not the Owner or Designated Manager of a pharmacy that, within the six (6) years immediately preceding the election, has undergone a re-inspection, as a result of deficiencies noted in an initial inspection, for a third time or more after the initial inspection.
Most meetings are virtual or hybrid. However, the Board meetings which take place at least 4 times a year are in-person meetings.
The College recommends that new application forms and documents are submitted each year reflecting how the applicant’s expertise aligns with the core competencies identified in that year’s Director Profile requirement.
All relevant information is contained on the College’s website and in the Election documents below:
As the regulator of pharmacists and pharmacy technicians in Ontario, the Board’s role is to serve and protect the public interest and to hold pharmacy professionals accountable to relevant laws, regulations, standards of practice, policies, guidance and the Code of Ethics. The Board is accountable to the public and does not represent or advocate for pharmacy professionals. That is the role of pharmacy associations.
Business Pressures
The College does not have oversight or any regulatory influence over pharmacy funding models as this is not within its legislated mandate. However, we will consider whether and to what extent funding plays a role in the delivery of safe and ethical care to patients.
No. The College will fully exercise its authority but will need to work with other system partners, including government, to address these issues.
Pharmacy professionals have raised similar issues previously. In response, in 2020 in collaboration with system partners, patients, pharmacy professionals and pharmacy ownership representatives, we developed a set of shared accountability principles.
Unfortunately, registrants continued to share with us that they are under increasing pressures from corporate ownership to focus on volume and financial targets over what is in the best interests of patients. It now seems clear that some of those who participated in the development of the principles are choosing not to apply them. We acknowledge that what we have done up until now is simply not enough. We are thankful to registrants for sharing their concerns with us.
Our new strategic plan includes a focus on addressing business interests that impede pharmacy professional autonomy and impact their wellbeing. As shared on this page, we are currently implementing several strategies as part of our strategic goal and zero-tolerance approach.
Most corporate-owned pharmacies have majority pharmacist ownership. While the College does not have authority over corporations, pharmacists–even those who act in their capacity as corporate directors rather than providers of patient care–are accountable to us.
We continue to explore what more we can do to assure ourselves and the public that no matter what ownership models are in place, pharmacy professionals have the autonomy to use their own knowledge, skills and judgment in the delivery of patient care.
No. Patients should trust in the safety and quality of the care provided by their pharmacy professionals. By addressing these pressures now, our goal is to ensure that quality is preserved, and all patient care is guided by the best interest of the patient at all times.
MedsCheck reviews can save lives and are an important part of a pharmacist’s role in helping patients take their medications safely. The service, when done properly and in accordance with program requirements, provides significant benefits to patients and to the health system.
Publicly funded programs are designed and managed by the Ministry of Health so changes to the MedsCheck program are not within the College’s authority.
However, registrants and pharmacies are required to comply with all relevant rules associated with these and other programs and must put patient needs first by practicing according to professional and operational standards and their ethical obligations. The College can take a role in ensuring compliance.
The College has heard from pharmacy professionals about ongoing concerns they have about pressures being placed on them to perform services that get in the way of their ability to provide the kind of care they want to provide to their patients. More significantly, we’ve heard that these pressures can impact pharmacy professionals’ wellbeing and place pressures on them to make decisions that prevent them from meeting their obligations as regulated pharmacy professionals.
Examples include the use of operational plans, volume targets or financial pressures that impede pharmacist autonomy to make decisions in the best interest of their patients.
No. If you wish to file a complaint or report information, please do so through our existing process. More information about complaints and reports are available on our website.
The tool is designed for pharmacy professionals to share their experiences with business pressures. This applies to all pharmacy professionals working in any practice environment, including independent pharmacies.
If you wish to share your information anonymously, submit this online form. While we will not be able to trace actual submitted forms to any sender, the College may be obligated to take action, such as an investigation, based on the information shared with us. If so, any information submitted may be shared with relevant parties. Please consider this when providing your information through this online tool.
Information shared by registrants may be used to:
- Identify new trends that OCP should be aware of so we can respond more quickly
- Identify specific pharmacies or pharmacy corporations where registrants are experiencing these pressures, and to understand the nature of those pressures more clearly
- Contribute to OCP’s actions towards specific pharmacies or corporations in response to concerns shared
- Identify gaps in understanding or communication so OCP can act more quickly to address them.
Cannabis
No. Under the federal Cannabis Act, the Access to Cannabis for Medical Purposes Regulations prohibit pharmacies (or any retail outlet) from dispensing cannabis for medical purposes.
A pharmacist cannot alter or repackage cannabis, or be responsible to obtain, possess, or distribute cannabis on behalf of a patient.
The holder of a federal license for sale for Cannabis for Medical Purposes must deliver directly to the patient, by mail and in the licensed processor’s original packaging, pursuant to an order from a health care practitioner.
Cannabis for recreational or non-medical purposes can only be distributed or sold through the online Ontario Cannabis Store or through privately run retail stores that have received a license from the Alcohol and Gaming Commission of Ontario.
Community pharmacists are not exempt from the methodologies of distribution of cannabis outlined in the legislation. For additional information, please contact Health Canada’s Cannabis Legalization and Regulation Branch (CLRB) at [email protected].
Pharmacists are expected to gather relevant information through dialogue with the patient to assess the appropriateness of a prescribed medication and to identify potential drug interactions. This may include asking about allergies, medical conditions, and lifestyle factors, such as the patient’s diet, alcohol, exercise, tobacco, and cannabis consumption.
As medication experts, pharmacists are in a unique position to support patients who consume cannabis, collaborating with other health care professionals as needed, to promote patient safety while striving to meet their health goals and desired treatment outcomes.
Pharmacists are medication experts and are often the most accessible healthcare providers for patients. Ontarians who consume or are considering consuming cannabis should expect their pharmacist to have the knowledge to help them make the best decisions about their care and to be able to effectively respond to their cannabis-related inquiries.
In addition to the opportunity to provide advice on cannabis use, there is also an opportunity to educate the public on harm prevention strategies to reduce possible risks associated with cannabis consumption and to identify and assist patients who are at risk of cannabis use disorder. Also, as a patient could acquire recreational cannabis without the intervention of a health professional and intend to consume it for medical purposes, pharmacists are in an ideal position to support patients in such circumstances.
No. Under the federal Cannabis Act, activities with cannabis are prohibited unless authorized by the Cannabis Regulations. It is prohibited to obtain, or offer to obtain cannabis by any method or process, including altering its chemical or physical properties. Any compounding activity involving cannabis would require enabling regulations or an exemption from the application of the Act by the Minister under subsection 140(1).
For additional information, please contact Health Canada’s Cannabis Legalization and Regulation Branch (CLRB) at [email protected].
Community Pharmacy Accreditation Renewal
The Director Liaison of the corporation and appointed Designated Manager must complete the pharmacy accreditation renewal process.
The individual who completed the pharmacy accreditation renewal can access and print the payment receipt and/or accreditation certificate from their online registrant profile. Please follow the below steps:
- Login to My Account
- Select “Accreditation Renewal”
- Click the “Payment List” button on the introduction screen
- Select the “Paid” tab.
The user will be taken to the payment list where both the receipt and accreditation renewal certificate are available. To print these documents, disable any popup blockers.
Director Liaisons and appointed Designated Managers can complete the renewal process using a desktop or laptop computer. The annual renewal process is not compatible with mobile phones.
Ensure you are using the most up to date version of either Internet Explorer 11, Microsoft Edge, Safari, Chrome or Firefox, and that you have installed Acrobat Reader on your computer. If you wish to print confirmation of completion, please ensure your printer can print from your web browser prior to starting the pharmacy accreditation renewal process.
Director Liaisons must declare that they will not allow business interests and management pressures to undermine their pharmacy’s ability to provide safe, quality care to patients as required by the Code of Ethics, Standards of Practice and Standards of Operations. This declaration aligns with one the College’s strategic goals to help ensure business practice do not compromise the health and well-being of pharmacy professionals or impede their ability to adhere to the Standards of Practice and Code of Ethics.
The Director Liaison will be asked to complete a declaration on the AIMS (Assurance and Improvement in Medication Safety) Program.
The AIMS declaration asks Director Liaisons to confirm their pharmacies fulfil the requirements of the AIMS Program, including the completion of the Pharmacy Safety Self-Assessment (PSSA).
The Director Liaison is accountable for ensuring their pharmacies are compliant with the requirements of the mandatory AIMS Program and that their pharmacy teams are using the AIMS Pharmapod platform to record incidents and good catches (near misses) to optimize patient outcomes.
Director Liaisons and appointed Designated Managers should set aside at least 10-15 minutes to complete their community pharmacy accreditation renewal. If there are changes to corporation or workplace information, you will be asked to update it. Director Liaisons will also be asked to complete declarations. These declarations should be read carefully before responding.
The community pharmacy accreditation renewal is comprised of three parts: corporate information renewal, pharmacy information renewal and payment. Each part must be completed to successfully renew your pharmacy’s license.
- Corporation Information Renewal: Verify or update corporation information. This information is only viewable by the Director Liaison of the corporation.
- Pharmacy Information Renewal: Verify or update pharmacy information.
- Payment: The College accepts credit card, cheque or money order.
Community Pharmacy Assessment
In order to ensure that the operations advisor knows which documents correspond to which type of activity, we ask that you please NAME the document attachments to indicate this clearly. For example: “Narcotic reconciliation August 2020,” “Mixture Listing Report” or “Blister pack label.”
We take patient privacy very seriously at the Ontario College of Pharmacists. All document submissions containing personal health information (patient name, etc.) are done via an encrypted web portal.
The web portal has end-to-end security, meaning all information is secure from the moment you send the email to the moment we receive it at the College, i.e., secure lifecycle management of information.
Redaction of patient information should be done according to your organization’s policies. Operations advisors do not need access to patient identities in order to perform the assessment. However, it is important for YOU to know the identity of your patients to provide any additional information during the assessment. If redacted copies are sent to OCP, you should keep a record of the patient’s name for your own use.
The Designated Manager of the pharmacy will be able to access the pharmacy assessment report after the assessment. Please do not respond to the action plans via email or encrypted email.
To access your assessment report:
- Go to https://members.ocpinfo.com.
- Enter your OCP username (OCP#) and password.
- Click on Pharmacy Assessment on the upper right side of the screen.
- Then click Pharmacy Assessment Report/Action Plan on the left-hand menu. This will bring you to the Quill site.
- Enter your OCP Username (OCP#) and password again. Please note: the initial sign in to the OCP portal is not case sensitive, but the Quill login is case sensitive.
- The assessment report can be accessed by clicking View Inspections
If the outcome of your assessment requires an action plan to be submitted, the action plan can be accessed by clicking View Active under Action Plan Summaries.
The operations advisor will review the documentation submitted. If clarifications or further information is needed, the Designated Manager will be contacted directly.
The Designated Manager should send in two complete reconciliations completed for narcotics, controlled drugs and targeted substances (one of which should be from at least 6 months ago). The reasons and investigations for any discrepancy found in the reconciliation is to be documented and available for assessment by the operations advisor. It is best if this documentation can be available on one page for each discrepancy or documented on the full count if it is clear. If your reconciliation documentation for submission exceeds 40 pages total, please contact your advisor directly to discuss what documents are required. All documents pertaining to reconciliation of controlled substances in the past two years must be available for the onsite or remote assessment.
If you keep individual pages for each controlled substances, send in reconciliation logs for five different narcotics (e.g. oxycodone), logs for five controlled drugs (e.g. amphetamine), and logs for five targeted substances (e.g. lorazepam). Include a description of how your process ensures that all controlled medication received and dispensed every six months have been reconciled with your current process. Please also ensure that all records of reconciliation are available for the onsite or remote assessment.
The type and amount of documents we are asking for will be clearly laid out in the attachment in the encrypted notification email sent to you by the College. Please review this carefully. This will differ depending on the types of services offered by your pharmacy. The purpose of asking for a variety of different documents is so that the operations advisor is able to get a good picture of the breadth of your operations and the types of services you manage. The documents and questionnaire will also enable your operations advisor to understand your policies and procedures.
The College is unable to accept mailed documentation. Please do not mail any documentation to the College. If you have unique circumstances around documentation submission please consult with your organization, if required, and discuss directly with your College advisor.
OCP is committed to providing the highest level of security, controls and integrity to support secure email encryption for information transfer. As such our email encryption service provider adheres to the following standards:
- Web Trust Certified
- PCI DSS Level 1 Certified
- Encryption Standards:
- RSA 2048-bit asymmetric encryption
- RSA PKCS cryptographic protocols; PKCS#1, #7, #10, #12
- AES-256 symmetric encryption
- SHA2 hashing algorithm
- ANSI X.509 certificates and certificate revocation lists
- IETF MIME and S/MIME email
When you first receive the encrypted email from the College, you will be asked to create an email encryption account with a username and password (details will be attached to your encrypted email). Once you have created this account, you are able to log-in at any time.
For all of the requested documentation, it is suggested you either scan the documents and save screen shots as PDF or take a picture and save as JPEG (picture). All of these types of documents can be ATTACHED to an encrypted email via the portal. Please ensure the quality of the picture is of good clarity and reasonable size so the operations advisor can read it easily.
PLEASE NOTE: compressed files are NOT permitted.
You will NOT have the ability to compose a new email in the encrypted account. Please REPLY to the original encrypted email that was sent to you, and add attachments as you normally would. There is a 35MB limit per email, so you may need to send multiple emails with attachments. This is perfectly acceptable.
Please also note that the encrypted email AUTO-DELETES after 30 days. If you do not submit your documentation within 30 days, you will need to email [email protected] to request a new link.
Please refer to the initial encrypted email sent to you as it specifies the date when you should be submitting the requested documents. Ideally, all documentation should be submitted approximately three to four weeks after you receive your assessment notification. This allows the operations advisor enough time to review the documents thoroughly and to contact you for clarification or for missing documents.
Complaints and Reports
Complainants are not parties to such disciplinary proceedings. They do not direct the prosecution or make submissions separate from the College’s submissions. However, it is possible that you may be called as a witness in the proceedings.
The decision of the Inquiries, Complaints and Reports Committee is not admissible in a civil proceeding pursuant to the Regulated Health Professions Act, 1991.
The College posts information about concerns that are relevant to a pharmacy professional’s suitability to practice on the Find a Pharmacy or Pharmacy Professional tool. The results of an investigation are public information when pharmacy professionals are ordered to complete a specified continuing education and remediation program, receive an oral caution from the Inquiries, Complaints and Reports Committee, or when specified allegations of professional misconduct or incompetence against the pharmacy professional are referred to the Discipline Committee.
Complaints are confidential and while the results of investigations remain on the pharmacy professional’s file, some investigation results are not available to the public if they are not relevant to the professional’s suitability to practice. You can learn more about the public register and what information is made public and not made public on our Public Information on Pharmacy Professionals page.
At any point after a complaint is filed, you can request to withdraw your complaint against the pharmacy professional. Withdrawal requests are reviewed by the Registrar, who determines whether it is in the public interest to grant the withdrawal of the complaint. If the Registrar is of the view that it is in the public interest to continue with the complaint investigation, the withdrawal request will be denied, the investigation will be completed, and the results of the investigation will be reviewed by the Inquiries, Complaints and Reports Committee.
Before filing a complaint, we encourage you to talk directly with your pharmacy professional and/or the manager of the pharmacy to address your concern, if you feel comfortable doing so.
Once you’ve made a complaint, any conversation you have with pharmacy staff should stay focused on your usual pharmacy care. We request that you and the registrant do not discuss the complaint further.
There is no time limit to filing a complaint. However, the College recommends you file your complaint as soon as possible so both parties are able to recall the events in question.
You do not need a lawyer. However, you can get legal advice or retain a lawyer to help you in the process if you want to.
College staff cannot direct a pharmacist or pharmacy technician to provide a specific treatment or service. We do not have jurisdiction to award damages or require a registrant to provide compensation. If you are seeking compensation for harm caused, you may wish to speak with a lawyer.
Controlled Substances, Buprenophine, Methadone
The College’s Opioid Policy specifies that pharmacists dispensing methadone for Opioid Agonist Treatment (OAT), also known as methadone maintenance treatment (MMT), must be in compliance with the Key Requirements for Methadone Maintenance Treatment (MMT) Dispensing Fact Sheet, which states:
“Community pharmacies must inform the College within seven days of starting to dispense MMT and of any changes in this information, using the approved Methadone Dispensing Notification Form.”
Notification is required to help the College fulfill its mandate to protect the public. All pharmacies undergo routine assessments every one to four years, depending on the activities performed at the pharmacy and the risk of harm those activities pose to the public. A community pharmacy dispensing methadone will be evaluated according to the operational assessment criteria more often than a pharmacy which does not.
Pharmacies dispensing methadone as an opioid analgesic for pain or buprenorphine/naloxone are not required to notify the College.
The College’s expectations for buprenorphine/naloxone dispensing for Opioid Agonist Treatment (OAT) can be found in the Opioid Policy. This includes practicing in accordance with CAMH’s Opioid Agonist Maintenance Treatment: A Pharmacist’s Guide to Methadone and Buprenorphine for Opioid Use Disorder.
Additional resources are available in the Opioid and Opioid Use Disorder Practice Topic such as the CRISM National Guideline for the Clinical Management of Opioid Use Disorder.
Although the Opioid Policy does not mandate formal training as a prerequisite for dispensing buprenorphine/naloxone, the College lists continuing education opportunities on its website as a tool to assist with professional development.
As with any medication, pharmacists are relied upon to ensure they have the necessary knowledge, skills and judgment to provide OAT in a safe and effective manner.
The College’s Opioid Policy sets out expectations for pharmacists dispensing all opioids, which includes methadone for pain or analgesia.
Pharmacists should adhere to the most recent clinical practice guidelines and the appropriate standards of practice to ensure best patient outcomes for individuals on opioid therapy. The Opioids and Opioid Use Disorder Practice Topic provides easy access to numerous resources to support pharmacy professionals in safe opioid dispensing.
Pharmacy professionals who accept prescriptions for any drug from an out of province prescriber should refer to the College’s Cross-Jurisdictional Pharmacy Services Policy. In addition, pharmacists should be familiar with the College’s Opioid Policy prior to dispensing any methadone prescriptions, and have the required reference Opioid Agonist Maintenance Treatment: A Pharmacist’s Guide to Methadone and Buprenorphine for Opioid Use Disorder (CAMH).
Collaboration with the prescriber may be required to ensure methadone prescriptions are written in accordance with Ontario MMT policies as well as the Narcotic Safety and Awareness Act requirements. If a pharmacy professional is unsure of the scope of practice and prescribing authority of another regulated health professional, they should contact the individual’s regulatory body for clarification.
There may be more than one way to dispense a prescription to ensure the patient receives the correct drug, dose and total quantity, such as in the situation you describe. The College cannot provide a specific answer on the action the pharmacist should take; it is the registrant’s responsibility to assess the prescription and decide the best course of action based on all the information at their disposal.
In situations where a specific dose or strength indicated on a prescription does not exist, is unavailable, or is not the most appropriate option for the patient, the pharmacist, in exercising their professional judgment, may choose to use available dosage forms to make up the prescribed strength/dose. Alternatively, the pharmacist may choose to contact the prescriber for clarification — either in writing or verbally — when presented with a prescription that is ambiguous or the intent of the prescriber is not clear.
Depending on the specific scenario, it may be important to ascertain if there was a clinical reason for prescribing a particular strength or dosage form. If there are different options, what does the patient prefer? Or, if the drug does not exist in a given strength, is it possible an error was made in the name of the drug or the dose?
When deciding whether or not it is appropriate to split the tablets prior to dispensing, pharmacy professionals should apply their knowledge to determine whether or not the medication is amenable to splitting (e.g. scored or not, accuracy of resulting dose, stability, tablet integrity) and keep the patient’s health outcomes, best interest and safety in mind.
Documentation of one’s rationale is important, in addition to documentation of dialogue with the patient, their understanding of the changes, and their informed consent. Pharmacy professionals are encouraged to reach out to their peers for timely guidance with practice-related questions as well, to leverage their practical experience and get advice on how they approach situations like this, assess the prescription, and exercise professional judgement.
Designated Manager
The DM has authority and accountability over decisions affecting the operation of a pharmacy and is responsible for effectively overseeing the day-to-day management of the pharmacy.
Regulations place the responsibility of maintaining the standards of accreditation on the owner and DM. To ensure staff engagement and understanding in maintaining these standards, the DM must provide access and orientation to the pharmacy’s policies and procedures and have systems in place to assess ongoing compliance. For example, policies and procedures should address how pharmacy staff are required to collect, use, protect, store and dispose of personal health information, especially when using technology to deliver patient care.
DMs must meet and maintain the Standards of Operation for the pharmacy, which include implementation of the College’s Assurance and Improvement in Medication Safety (AIMS) program to support patient safety and continuous quality improvement. Internal policies and procedures must be regularly reviewed and updated in response to changes in the practice environment (i.e., public health emergencies, amendments to legislation, new equipment, etc.).
The Professional Supervision of Pharmacy Personnel Policy further describes the role of the DM in human resources management, such as ensuring:
- adequate supervision of both professional and lay staff to perform their assigned duties
- controlled acts are only performed by regulated staff or under delegation.
- workflow processes are sound, robust and consistently followed to optimize the delivery of professional services
- staffing supports registrants in meeting the standards of practice for patient care at all times
A comprehensive list of obligations can be found under the Role of Designated Manager section in the Application for a Certificate of Accreditation and/or Change of Designated Manager Form.
Pharmacist owners/directors automatically assume responsibility in the absence of a DM. If there is no DM on a pharmacy’s record with the College, a representative from Pharmacy Applications & Renewals will follow up with the owner/director liaison. Compliance with the Drug and Pharmacies Regulation Act, which requires a DM to be on the public register, is a requirement for the annual renewal of the pharmacy’s certificate of accreditation.
Nothing prohibits a pharmacist from acting as the DM at more than one community pharmacy. It is the pharmacist’s responsibility to ensure they are capable of fulfilling their obligations at every pharmacy where they hold this designation.
The College does not stipulate the number of hours a DM must work at their pharmacy nor set out time limits for absences. Rather, the responsibility is on the DM to determine the amount of time they need to be present to fulfill their obligations.
The DM is responsible for the overall day-to-day operation of the pharmacy whether they are physically present or not. In the event of an extended period of absence for any reason, it is up to the registrant to decide if they can continue to meet their obligations and duties as DM during this time or whether another pharmacist should take on this role in the interim. Whenever there is a change in the DM, the pharmacy owner must file notice of the change with the College.
Dispensing Prescriptions
Regular e-mail (as opposed to a secure healthcare portal) is not a secure medium for prescription transmission.1 In addition to cybersecurity risks, when a prescriber e-mails a prescription directly to a patient, it increases the likelihood of fraud and duplication of the prescription. Therefore, unsecured email is not an acceptable mechanism for transmitting prescriptions, especially for monitored drugs and those listed under the Controlled Drugs and Substances Act (CDSA).
Upon receiving a prescription via unsecured email, pharmacists are encouraged to contact the prescriber to verify its authenticity and collaborate with them and the patient to determine the best method of transmitting a prescription from the prescriber directly to the pharmacy via established channels (e.g., fax, verbal confirmation, secure electronic transmission).
There may be times when a pharmacist needs to use their professional judgement when accepting an e-mailed prescription – for example, when a prescriber cannot be reached and the patient is at risk of going without necessary medication. In these cases, pharmacists should exercise their professional judgment in determining whether to dispense the prescription and follow-up with the prescriber at the earliest available time. Prescribers should also seek guidance from their regulatory College.
Reference
1. Information and Privacy Commissioner Ontario. Fact Sheet: Communicating Personal Health Information by Email (2016). Retrieved at https://www.ipc.on.ca/wp-content/uploads/2016/09/Health-Fact-Sheet-Communicating-PHI-by-Email-FINAL.pdf.
The requirements for a prescription to be “signed” are established in the federal regulations relevant for the schedule of the drug. There is nothing in the regulations mandating the use of a “wet” signature (e.g., “pen-and-ink” signature). As explained in the related 2014 article, Navigating Electronically Generated Prescriptions, “Health Canada has broadly defined ‘signing’ as whatever is determined to be necessary to authorize and validate the order”.
The College’s Position Statement Authenticity of Prescriptions using Unique Identifiers for Prescribers describes three examples of distinct authorization methods:
- a traditional pen-and-ink signature, or
- an electronically captured image of a unique signature (generated on a signature pad), or
- a unique prescription authorization process which ensures that the prescriber has reviewed and authorized each individual prescription
There may be many different technologies available to prescribers, especially as computerized order entry and electronic prescriptions become increasingly common, to implement a unique authorization process and generate an order without an actual image of a signature.
Dispensers should review the Position Statement in its entirety, especially the guidance provided in the section “The Responsibility of the Registrant”. If the pharmacist decides that they need to verify the authenticity of the order, this may be done verbally or in writing (via fax). Prescribers should seek guidance from their regulatory College.
The College does not maintain information on the scope of practice of practitioners other than pharmacy professionals in Ontario. Questions about the scope of practice and prescribing authority of another health professional should be directed to their regulatory body. Collaboration with the practitioner is also encouraged, as self-regulated health professionals should be aware of – and practicing within – their legal scope of authority and the policies and guidelines of their College. This topic was covered in the Summer 2017 Pharmacy Connection article Prescriptions from Other Healthcare Professionals.
The Cross-Jurisdictional Pharmacy Services Policy explains that dispensers may accept a prescription written by a prescriber licensed in any province or territory of Canada. The Drug and Pharmacies Regulation Act (DPRA) defines “Prescriber” as “a person who is authorized under the laws of a province or territory of Canada to give a prescription within the scope of his or her practice of a health discipline”. An out-of-province prescriber is registered and regulated by their province’s (or territory’s) health disciplinary college. Therefore, the regulations and corresponding scope of practice of the same health discipline in Ontario cannot be applied to an out-of-province practitioner. Similarly, a pharmacist licensed in another province whose scope includes prescribing authority (for example, to initiate treatment for minor ailments or to renew a prescription) is considered a prescriber for the purposes of the Act.
The Regulated Health Professions Act has provisions to allow a regulated health professional to delegate their authority to perform a controlled act to a person (regulated or not) who does not have that authority. Delegation of authority can be implemented by use of a Direct Order or a Medical Directive. In this case, the controlled act is “prescribing”.
The College’s Medical Directives and the Delegation of Controlled Acts Policy sets out the expectations for registrants who are considering authorizing or implementing delegated acts.
A prescription issued pursuant to a directive must include the:
- Name of prescriber (delegator; authorizer of the directive)
- Name and signature/unique authorization of the person issuing the prescription (delegate; implementer of the directive)
- Name and number of the medical directive
- Contact information for the authorizer and implementer
The prescriber’s name (delegator; authorizer of the directive) should be recorded on the dispensing record as they are ultimately responsible for the prescription, even though they have delegated the authority to perform the act to someone else to implement via a directive.
Should there be questions about the prescription, registrants should contact the implementer. If the questions cannot be resolved, the prescriber would be contacted for clarification. Where requested, a copy of the directive may also be forwarded to the pharmacy.
More information can be found in the Health Professions Regulators of Ontario Interprofessional Guide, FAQ, and templates.
Documentation, Record Keeping and Privacy
Please refer to the Record Retention, Disclosure, and Disposal Guideline and the Record Keeping and Scanning Requirements Fact Sheet.
Regulations under the Drug and Pharmacies Regulation Act require pharmacies to make and retain a scanned copy of all original prescriptions. However, the decision to destroy paper-based records after they have been scanned is at the discretion of the Designated Manager who should evaluate the backup processes in place to safeguard records for the required timeframe.
In terms of the timeframe, all prescription records in the pharmacy’s possession, scanned or in hardcopy form, are subject to the retention period of a minimum 10 years after the last date of service provided to the patient. Since records cannot be destroyed until at least 10 years after the patient has ceased to use a pharmacy’s services (notwithstanding the provision for children under 18 years of age), the date of when a record can be destroyed cannot be pre-determined.
Also, the patient record must be maintained as a whole, therefore dispensing records (hard copies) and prescriptions (originals or scanned originals) cannot be singled out for destruction. If the patient continues to use the services of the pharmacy, the patient record would need to be retained on file for an indefinite period of time (i.e., forever).
Pharmacies and pharmacy professionals in Ontario have responsibilities to safeguard the personal health information of their patients as health information custodians under the Personal Health Information Protection Act (PHIPA). As PHIPA establishes the mandate of the Information and Privacy Commissioner of Ontario, please refer to Information and Privacy Commissioner (IPC) of Ontario Fact Sheet – Obtaining Personal Health Information About a Deceased Relative. Additional information can be found on the IPC website.
The College’s Releasing Personal Health Information Fact Sheet describes some of the most common disclosures permitted by pharmacists/pharmacies as health information custodians under Ontario’s Personal Health Information Protection Act (PHIPA). For example, information may be disclosed when a pharmacy professional is presented with a warrant, court order, production order, or under authority established in legislation, such as the Coroners Act and the Missing Persons Act. There may be other legislation—such as Canada’s Criminal Code or the province’s Child, Youth and Family Services Act — which may prevail over PHIPA.
The Information and Privacy Commissioner of Ontario, who provides oversight of Ontario’s access and privacy laws including PHIPA, has additional resources to guide registrants in their decisions surrounding personal health information, including:
As always, supporting documentation is essential as evidence of your decision-making process and rationale. If a registrant is uncertain about their obligations after reviewing the appropriate resources and legislation, they may wish to obtain independent legal advice or consult with their corporation’s privacy officer.
The “transfer of records” process is not the same as a prescription transfer (as permitted by the Drug and Pharmacies Regulation Act). When closing a pharmacy, all patient files and prescription records are “transferred” or relocated to the receiving Designated Manager (DM)/owner, and the prescriptions will then belong to the receiving pharmacy. Exactly “how” the records are entered or merged into the receiving pharmacy’s software system is sorted out by the DMs and the vendor(s). Ideally, it should be seamless to the patient as they must continue to be able to access their personal information.
Pharmacies and pharmacy professionals participating in the closure of a pharmacy should review the College’s closing a pharmacy webpage. Additionally, the College’s Record Retention, Disclosure and Disposal Guideline can provide additional information on the management of patient records.
Please refer to the Releasing Personal Health Information Fact Sheet, which describes a few of the more common disclosures permitted by health information custodians, which pharmacists are, under the Personal Health Information Protection Act (PHIPA). You can also to refer to the legislation directly for complete details or contact the Information and Privacy Commissioner of Ontario.
Regarding requests from health regulatory colleges in Ontario, all colleges – including the College – must follow the same Health Professions Procedural Code (set out in the Regulated Health Professions Act). Colleges also establish Rules of Procedure, as any hearings in Ontario must meet the standard for procedural fairness mandated by the Statutory Powers Procedure Act. You could reach out to the investigator directly regarding questions about their rules of procedure.
Specific to fentanyl, please refer to the Patch-For-Patch Fentanyl Return Program Fact Sheet. Regulation 305/16 under the Safeguarding Our Communities Act (Patch for Patch Return Policy) has specific provisions for dispensers providing information to law enforcement. Please refer to section 4(3) under ‘Contingency Plan’ in the regulations.
Emergency Assignment Registration
As a supervising pharmacist, you should assess the EA registrant’s competence by considering their experience practising in their full scope in Ontario (or a similar jurisdiction), and by initially observing their performance in practice. Based on your assessment, you and the EA registrant should discuss and agree on the types of practice activities they may perform independently, and when and how you will be consulted about practice matters. More information about the expectations for pharmacists supervising EA registrants and the documentation requirements are on this guideline document.
If you will be supervising a pharmacist (EA), you may wish to use the PACE assessment criteria, the community pharmacist practice assessment criteria or the hospital, family health team, and long-term care pharmacist practice assessment criteria as a guide to assessing their readiness for independent practice.
If you will be supervising a pharmacy technician (EA),you may wish to use the community and hospital pharmacy technician practice assessment criteria as a guide to assessing their readiness for independent practice.
To pursue full registration as a pharmacy technician in Ontario, you will need to follow the entire registration pathway that is relevant to you. This will begin with opening an online file, paying the initial application fee, and submitting all required supporting documentation.
All registrants must have valid personal professional liability insurance coverage when they submit their application to OCP and while they are registered with OCP.
OCP does not endorse one insurance provider over another. Since every provider’s insurance package differs, you should check with your insurance provider to confirm that your current insurance policy covers you while you are registered and wherever you may be practising as an EA registrant before you submit your application. If your current insurance policy does not provide you with full coverage as specified in the current By-Laws, you will need to obtain the insurance coverage to meet this registration requirement.
While registered as a pharmacy technician (EA), you may—and are encouraged to—continue your pharmacy technician registration process by taking the Jurisprudence, Ethics and Professionalism exam and the PEBC Qualifying Exam. If you attempt PACE while registered as a pharmacy technician (EA), your PACE assessor must still directly supervise and observe your practice.
If you meet all of the pharmacy technician registration requirements while registered as a pharmacy technician (EA), you may apply to register as a pharmacy technician. Once you have fully registered as a pharmacy technician, your EA certificate of registration will expire 60 days after it was last issued.
Once EA registration has ended, you will revert to your former intern status and scope of practice, and be required to meet all of the registration requirements for final registration as a pharmacist. As an intern, you must practise under appropriate supervision.
The expiry date of your intern certificate of registration will not change. If your certificate of registration is about to expire while you are practising as a pharmacist (EA), you should request an extension of your intern certificate from a panel of the Registration Committee.
While registered as a pharmacist (EA), you may—and are encouraged to—continue your pharmacist registration process by preparing for and attempting PACE, and by taking the Jurisprudence, Ethics and Professionalism exam and the PEBC Qualifying Exam. If you attempt PACE while registered as a pharmacist (EA), your PACE assessor must still directly supervise and observe your practice.
If you meet all of the pharmacist registration requirements while registered as a pharmacist (EA), you may apply to register as a pharmacist. Once you have fully registered as a pharmacist, your EA certificate of registration will expire 60 days after it was last issued.
If you moved from Part A to Part B and had provided patient care within the past three years, you are eligible to register as a pharmacist (emergency assignment).
If you have been a Part B pharmacist for greater than three years, you will not be eligible to register as a pharmacist (EA).
Expanding Scope of Practice
The OCP Board determined that mandatory learning for certain expanded scope activities will help ensure a consistent level of knowledge, skills and abilities among pharmacy professionals. The scope activities that require mandatory learning may pose a higher risk to the public if not managed appropriately.
Pharmacy professionals who choose to provide any services that require mandatory learning will be required to complete a Declaration of Completion of Learning to indicate they have the knowledge and skills to safely deliver these services.
Yes. At its December 8, 2025 meeting, the OCP Board determined that, once in effect, the following expanded scope activities require mandatory learning to support safe patient care. Mandatory learning is only required if a pharmacy professional chooses to provide any of the services below.
For pharmacists:
- Assessing and prescribing for sore throat (acute pharyngitis)
- Assessing and prescribing for shingles (herpes zoster)
- Assessing and prescribing for swimmers’ ear (otitis externa)
- Providing injectable partial opioid agonists and antagonists (specifically, buprenorphine)
Learning objectives for the above activities will be developed, validated and presented to the Board at their March 2026 meeting. The Board-approved learning objectives will be publicly consulted on in spring 2026 and be used to help identify appropriate courses/learning tools that meet the established learning objectives.
For pharmacy technicians:
- Administering vaccines listed in Schedule 3 of Ontario Regulation 256/24 under the Pharmacy Act, 1991.
Requiring pharmacy technicians to complete and maintain up-to-date CPR and First Aid training standardizes expectations among pharmacy professionals who are administering injections. The requirement for pharmacy technicians who administer injections to complete mandatory CPR and First Aid training will be publicly consulted on in January 2026.
The College’s role is to ensure that Ontarians receive safe pharmacy care from qualified pharmacy professionals.
The College’s mandate – which is set out in law – requires the College to meet the “objects” set out in section 3 of the Regulated Health Professions Act, 1991. The College has no role in compensating pharmacy professionals. The provincial government funds Ontario’s public healthcare programs and pharmacy employers establish compensation for their employees.
Expanded scope was introduced by the provincial government to provide long-term support to Ontario’s healthcare system. The provincial government has the power to amend existing laws and the Minister of Health directed the College to draft amended regulations under the Pharmacy Act to expand pharmacy professionals’ scope of practice. The College is responsible for ensuring any changes to pharmacy professionals’ scope of practice are implemented safely.
Providing these proposed additional services is a choice, not a requirement. Each pharmacy professional – pharmacists and pharmacy technicians – practices with professional autonomy.
Designated Managers must determine whether the pharmacy has the capacity to meet the Standards of Operations for Pharmacies to support safe patient care. One of the standards in the Standards of Operation for Pharmacies is: “The pharmacy has an adequate number of qualified and trained staff to maintain the accepted standards of professional practice, and to deliver safe and effective patient care.”
The College’s Board of Directors supports progress on addressing business pressures while also responding to government direction regarding expanded scope. The College’s continued work on addressing business pressures can be found on our Business Pressures in Pharmacy Practice webpage.
Taking on additional scope of practice activities is a choice, not a requirement. Having the legislative authority to prescribe for minor ailments does not mean all pharmacists must offer to provide this service. If a pharmacist does choose to take on new expanded scope activities, assessing and optimizing their pharmacy’s workflow and the role/responsibilities of other staff (e.g., pharmacy technicians, pharmacy assistants) can help pharmacists focus on clinical patient care.
At this time, the Ministry of Health has not moved forward with communicating a diagnosis as a proposed expanded scope activity for pharmacists. The College continues to share with the Ministry of Health the importance of pharmacists having the authority to communicate a diagnosis as scope of practice expands.
The Minister of Health requested a recommendation from the College in 2023 for additional minor ailments that pharmacists can assess and prescribe for. A list of minor ailments was developed by the College, informed by an expert advisory group, and submitted to the Minister of Health for consideration. In September 2025, the Ministry of Health officially requested that additional minor ailments be added to the scope of pharmacy practice.
The College is working with the Ministry of Health and system partners to determine an appropriate implementation date, ensuring that the expansion of scope activities can occur safely and that any necessary safeguards are implemented. Currently there is no change to the existing scope of practice for pharmacists and pharmacy technicians.
Fitness to Practise
The Health Professions Procedural Code, being Schedule 2 of the Regulated Health Professions Act, 1991, defines the term “incapacitated” as meaning that a registrant is suffering from a physical or mental condition or disorder that makes it desirable in the interest of the public that the registrant’s certificate of registration be subject to terms, conditions or limitations, or that the registrant no longer be permitted to practise.
A registrant with a physical or mental disability that has been properly addressed may not necessarily meet the definition of incapacitated; for example, a person who uses a wheelchair in an accessible workplace, or a person who has a mental health or substance use condition who takes appropriate measures to manage their condition may not meet the definition.
Incapacitated registrants may suffer from:
- Physical illness
- Mental illness
- Mood disorders
- Substance use disorder
- Certain physical, cognitive or sensory disabilities
- Other
Warning Signs
Many professionals who suffer from substance use challenges or who have an emotional or psychiatric disorder may experience various levels of deterioration in different facets of their personal lives. They are often still able to function professionally when other parts of their lives are not functioning, as work is often the last place to deteriorate — especially if the incapacity involves drug use. As a result, a person’s incapacity may have already escalated to a significant degree before it is observed in the workplace. The following are some typical, well-documented, warning signs for behaviours of a registrant who maybe incapacitated:
Physical changes
- Change in appearance/poor hygiene
- Tired appearance/insomnia
- Frequent shaking and/or sweating
- Loss of appetite/weight loss
- Slurred speech
Behavioural Changes
- Mood swings
- Memory loss or blackouts
- Withdrawal from friends and social activity
- Extreme anger, mistrust, anxiousness, depression, irritability
- Frequent work breaks
- Denial of having “problems” or need to be helped
Performance Changes
- Increased disorganization
- Increased number of prescription errors
- Increased number of customer complaints
- Frequent absences
- Lack of concentration or focus
Narcotic Shortages of Narcotics or Controlled Substances (in the case of substance use challenges)
- Shortages associated with the registrant ’s work schedule
- The registrant asks to have special narcotic responsibilities
- The registrant volunteers/asks to work shifts when the fewest staff are available
General Pharmacy Practice Questions
These are two distinct factors which must be considered independently in a scenario where the pharmacy professional is both dispensing and administering a substance.
“Schedules” are included in legislation to provide details (such as lists and tables) that aren’t suitable for including in the main content of the Act or Regulation. There are four schedules in O. Reg. 256/24 under the Pharmacy Act:
- Schedule 1: Injected substances
- Schedule 2: Inhaled substances
- Schedule 3: Vaccines
- Schedule 4: Drugs — minor ailments
In terms of scope of practice, pharmacists are authorized to administer a substance in Schedule 1 by injection, a substance in Schedule 2 by inhalation, or a vaccine in Schedule 3. (Schedule 4 relates to pharmacist prescribing and not to administration.)
Separately and distinctly, O. Reg. 264/16 under the Drug and Pharmacies Regulation Act requires pharmacies to follow the conditions of sale for a drug according to the NAPRA National Drug Schedules:
- Schedule I: Drugs require a prescription; only available from the dispensary
- Schedule II: Drugs do not require a prescription but do require intervention from the pharmacist; available from an area of the pharmacy where there is no opportunity for patient self-selection
- Schedule III: Drugs do not require a prescription, but a pharmacist or intern must be available for consultation; available for self-selection from the professional products area (within 10m/30ft of the dispensary)
- Schedule U: Drugs do not require a prescription or professional supervision; available from any retail outlet
For additional information please refer to:
Having a degree in pharmacy is one of several requirements needed for a certificate of registration, in order to practice as a licensed pharmacist in Ontario. It does not, in itself, confer the right to practice pharmacy. Should an individual wish to make reference to their educational credentials, this information should be presented in a manner that is not ambiguous, confusing, or misleading to the public. OCP has not established any additional policy or guideline restricting how a degree or credential may be used, however, it must be evident whether an individual is licensed by the College and able to practice pharmacy, or not.
Pharmacy graduates who possess a PharmD (Doctor of Pharmacy) degree should be aware that use of the title “Doctor” (or an equivalent abbreviation, such as Dr.) is restricted by the Regulated Health Professions Act, Section 33. A pharmacist cannot use this title in the course of providing, or offering to provide, health care to an individual. The regulated health professionals who may use this title in practice are defined in the Act. Pharmacists should be mindful when referring to their PharmD degree of the potential for misinterpretation by a patient that the individual is, for instance, a Medical Doctor (MD).
Once registered with the College as a pharmacist, they are permitted to use the title of “Pharmacist” or an equivalent abbreviation, such as the designation “RPh” (approved by Council in 2003). Section 10 of the Pharmacy Act also restricts the use of the titles “Apothecary” and “Pharmaceutical Chemist” to registered pharmacist registrants of the College. Similarly, “Pharmacy Technician” (and its abbreviation RPhT) became a restricted title under the Pharmacy Act in December 2010. All other titles – such as Certified Pharmacy Technician (in use prior to 2008) – must no longer be used. This does not prevent someone from indicating their past achievement of passing the College’s certification exam, such as on a résumé, however the title of “Pharmacy Technician” cannot be used in practice unless they are registered as registrants of the College.
Providing a copy of the original with the transfer would be considered a best practice in the interest of patient safety. Unlike a refill, the logged prescription has never been dispensed and has not gone through the same complete checking process. This is the same reason registrants within their own practice site should be retrieving or viewing the original hardcopy before a logged prescription is dispensed.
The Code of Ethics also expects that when a patient moves from one healthcare provider to another, the relevant information is provided to the receiving healthcare provider, to ensure safe and effective transition of care
It is the professional responsibility of a pharmacist to self-assess their competency and ensure they possess sufficient knowledge, skills and judgment to safely dispense any medication. A pharmacist is expected to undertake education or training as necessary to address any knowledge gaps so that they are able to effectively assess the patient and evaluate the appropriateness of the prescribed therapy. Pharmacists are reminded that they are responsible for providing the patient with relevant and sufficient information about the medication, including how to manage potential risks associated with use, and for providing appropriate follow-up. Given the serious safety concerns identified with the use of Mifegymiso®, pharmacists should report any cases of serious or unexpected side effects to Health Canada’s MedEffect Canada adverse reaction reporting system or to the manufacturer. Additional resources are available on the Linepharma website. Also, the Canadian Pharmacists Association has published a checklist for pharmacists and the Society of Obstetricians and Gynecologists of Canada offers a training program.
As explained in the Pharmacist Prescribing: Initiating, Adapting and Renewing Prescriptions Guideline, pharmacists have the independent authority to initiate treatment for smoking cessation with buproprion or varenicline under O. Reg 256/24 of the Pharmacy Act.
The regulations do not require a pharmacist to complete specific training prior to initiating treatment for smoking cessation. Similarly, OCP does not require or endorse a particular program you may wish to take. As with any patient care scenario, pharmacists are relied upon to practice within the limits of their competence, and to obtain the knowledge and skills necessary to carry out their professional duties.
However, you may be required to complete a certification course for the purposes of obtaining reimbursement for providing a specific smoking cessation service offered through a patient’s third-party insurer or the Ministry of Health.
The federal Precursor Control Regulations sets a maximum amount of pseudoephedrine and/or ephedrine that may be sold per package*, but not a limit on the total amount or quantity of pseudoephedrine and/or ephedrine that may be sold per transaction. Nevertheless, registrants have a professional and ethical obligation to prioritize the safety and best interests of the patient and the public.
If diverted to the illicit market, pseudoephedrine or ephedrine can be used to illegally produce D-methamphetamine (“meth”, “crystal meth”), which poses a significant risk to public health and safety. Given these risks, registrants must remain vigilant and exercise professional judgment when determining the quantity to sell, considering the patient and circumstances, to prevent potential substance misuse. For example, a registrant may restrict or limit the quantity sold to an individual if the request appears inappropriate based on their assessment.
*Based on maximum amount of pseudoephedrine and/or ephedrine base per package (e.g., maximum for pseudoephedrine base is 3 grams = 3.659 grams pseudoephedrine HCL). For conversion factors, refer to: https://www.deadiversion.usdoj.gov/quotas/conv_factor/index.html.
It depends on the product’s classification at the federal level:
a) Natural Health Products (NHPs) containing pseudoephedrine and/or ephedrine (i.e., products assigned a NPN by Health Canada) are not included in the NAPRA National Drug Schedules (NDS) and therefore are not subject to the related NAPRA NDS conditions of sale. However, they are still defined as ‘drugs’ by Ontario regulations and Health Canada’s Ministerial Order sets out retail sale prohibitions for these products.
Designated Managers must ensure that these products are only available for sale while the pharmacy is in operation (i.e., when a pharmacist is physically present in the pharmacy, providing direct supervision to personnel).
Health Canada’s Order outlines the following conditions-of-sale restrictions:
- Non-combination (“single entity”) NHPs containing only pseudoephedrine and/or ephedrine as medicinal ingredient(s) can only be sold by a pharmacist or a person working under the supervision of a pharmacist.
- Combination NHPs containing pseudoephedrine and/or ephedrine plus one or more other NHP ingredients (substances in Schedule 1 of the Natural Health Product Regulations) can be sold only if a pharmacist is available to assist consumer prior to purchase if requested.
These restrictions also apply to remote dispensing locations (RDLs) and online sales. See related FAQ on “Can products containing pseudoephedrine or ephedrine only be sold in pharmacies?”
b) Health Canada’s Order does not apply to non-prescription drugs (NPDs) containing pseudoephedrine and/or ephedrine and other drugs (i.e., products assigned a DIN by Health Canada).
As Ontario regulations require pharmacies to follow the conditions of sale for a drug according to the NAPRA National Drug Schedules (NDS), if the NPD contains:
- Schedule II or III drugs
• The ingredient with the most stringent NAPRA NDS conditions for sale determines whether pharmacist intervention is required; and
• The Supplemental Standards of Practice for Schedule II and III Drugs apply. - Unscheduled drug(s)
• They are not subject to the regulations and may be sold by any retail outlet.
In any case, the Designated Manager can choose to implement procedures for a pharmacist to intervene in the sale of any NHP or NPD containing pseudoephedrine and/or ephedrine, if there are concerns about misuse or diversion.
It depends on the product’s classification at the federal level:
a) Natural Health Products (NHPs) containing pseudoephedrine and/or ephedrine (i.e., products assigned a NPN by Health Canada) are beyond the scope of the NAPRA National Drug Schedules (NDS) and therefore are not subject to the related NAPRA NDS conditions of sale. However, pseudoephedrine and ephedrine are still defined as ‘drugs’ by Ontario regulations, which limits their sale to accredited pharmacies in Ontario. In addition, Health Canada’s Ministerial Order sets out retail sale prohibitions for these NHPs across the country.
Designated Managers must ensure that these products are only available for sale while the pharmacy is in operation (i.e., when a pharmacist is physically present in the pharmacy, providing direct supervision to personnel).
Health Canada’s Order outlines the following conditions-of-sale restrictions:
Non-combination (“single entity”) NHPs containing only pseudoephedrine and/or ephedrine as medicinal ingredient(s) cannot be accessible to the public for self-selection (e.g., must be kept behind the counter).
Combination NHPs containing pseudoephedrine and/or ephedrine plus one or more other NHP ingredients (substances in Schedule 1 of the Natural Health Product Regulations) can be accessible to the public in the self-selection area of the pharmacy.
These restrictions also apply to remote dispensing locations (RDLs) and online sales. See related FAQ on “Who can sell products containing pseudoephedrine or ephedrine in the pharmacy.”
b) Health Canada’s Order does not apply to non-prescription drugs (NPDs) containing pseudoephedrine and/or ephedrine and other drugs (i.e., products assigned a DIN by Health Canada). Ontario regulations require pharmacies to follow the conditions of sale for a drug according to the NAPRA National Drug Schedules (NDS).
Therefore, if the NPD contains:
Schedule II or III drug(s): The ingredient with the most stringent NAPRA schedule determines its conditions for sale (e.g., if the NPD contains Schedule II and III drugs, then Schedule II applies).
Unscheduled (“Schedule U”) drug(s): They are not subject to the regulations and may be sold by any retail outlet.
In any case, the Designated Manager can choose to keep any NHP or NPD containing pseudoephedrine and/or ephedrine behind the counter if there are concerns about misuse or diversion.
Hospital Pharmacy Assessment
For administrative matters, such as scheduling, pre-visit information and submission portal guidance, please email [email protected]
For issues with accessing Quill, please email [email protected]. Please note that the username and password should be accessible from the hospital’s designated contact.
For questions related to a recent hospital operational assessment or an inquiry about any hospital operational criteria, please email your designated hospital operations advisor. If you do not know who your dedicated advisor is, please email [email protected].
For questions on general pharmacy practice, please email [email protected]. Response times may vary depending on volume and the nature of the question.
Following an alleged incident of chemotherapy underdosing in Ontario and New Brunswick hospitals, the Ministry of Health commissioned Dr. Jake Thiessen to do an independent review. Among his recommendations were one suggesting that the College license and inspect all pharmacies operating within Ontario’s clinics or hospitals. In response, the government introduced Bill 21: Safeguarding Healthcare Integrity Act, which amended the DPRA.
On August 1, 2016, new regulations to the Drug and Pharmacies Regulation Act (DPRA) gave the Ontario College of Pharmacists the authority to licence and inspect hospital pharmacies. Expectations of practice for pharmacy professionals were not changed.
Hospital pharmacies are now issued certificates of accreditation, which must be renewed on an annual basis. In addition, hospital pharmacies undergo regular assessments every one to two years.
As outlined in your prior notice letter, as the designated contact, you are required to give an update in Quill regarding items that were outside of compliance during the last assessment. An update must be completed two weeks ahead of your scheduled hospital operations assessment date. This allows the operations advisor enough time to review thoroughly and to contact you for clarification. When responding to each item, please include a timeline for anticipated completion for all criteria, as well as the current status of the criterion.
To access this portal please use the link provided below:
https://hhapps.ocpinfo.com
The user # and password will have been assigned to you from your previous assessment. If you are having trouble with this process, please email [email protected].
Hospital Pharmacy Operation
Registrants must only engage in restocking of drugs under the authority of the accredited hospital pharmacy where the pharmacy has established this practice as permitted through the DPRA, O. Reg. 264/16, s.20.
With respect to the integrity of the drug, it is the College’s expectation that the accredited hospital pharmacy ensures that the drug has been in the possession of a licensed healthcare professional at all times (from time of dispensing to time of return to the pharmacy). If the drug has left the possession of a licensed healthcare professional, even for a short period of time, the drug integrity cannot be verified and restocking is not permitted. The licensed healthcare professional who has been in possession of the drug should confirm that the drug has been stored in accordance with the manufacturer’s requirements (e.g. appropriate temperature, etc.).
Internet Pharmacies
Pharmacy websites in the United States are reviewed and verified by the National Association of Boards of Pharmacy through its Verified Internet Pharmacy Practice Sites™ (VIPPS™) program. Please visit their website for more information.
The rules for operating an online pharmacy may vary in other provinces or territories. We encourage you to contact the local pharmacy regulatory authority directly for verification.
No. Ontario pharmacies can only dispense prescriptions authorized or signed by a prescriber licensed in a Canadian province or territory.
All pharmacies accredited by the Ontario College of Pharmacists (College) are “bricks and mortar” pharmacies within the province. These pharmacies may sell medications over the internet according to the College’s policy on Operating Internet Sites. This policy requires the pharmacy’s website to have its accreditation (license) number and the name of the Designated Manager so it can be verified on the College’s public register.
Avoid buying health products from questionable websites and verify that the pharmacy is legitimate.
Look for health products that have been authorized by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Medicine Number (DIN-HM). Consumers can also check whether products have been authorized for sale by searching Health Canada’s Drug Product Database, Licensed Natural Health Product Database or Medical Devices Active Licence Listing (MDALL).
You can also check Health Canada’s Recall and Safety Alerts Database for advisories on illegal health products that have been found in Canada.
A further source of reliable guidance on safe online pharmacies is the Alliance for Safe Online Pharmacies (ASOP) which is active in numerous jurisdictions internationally, including Canada.
Health Canada and the National Association of Pharmacy Regulatory Authorities (NAPRA) also offers guidance to help consumers choose a safe online pharmacy.
Jurisprudence, Ethics and Professionalism Exam
If you experience any technical difficulties at any time during the exam, notify the Prometric proctor immediately so that the issue can be addressed and resolved. If the technical issues cannot be resolved and cannot be reported on the post-exam survey, contact the College directly as soon as possible to report your issue. Prometric will notify the College of any incidents that it becomes aware of, and the College will follow up accordingly.
If you have questions about the Jurisprudence, Ethics and Professionalism exam, e-mail [email protected].
If you know that you will not arrive until after your scheduled appointment time, follow the instructions for late arrivals/set up on the Prometric Scheduling Confirmation Email.
If you believe, for whatever reason, that you are unable to do your best on the day of the exam, you are encouraged not to attempt the exam. You must inform the College of your decision not to take the exam in writing at [email protected] before the exam begins. You must also cancel your appointment with Prometric. For more information, please see information about withdrawals on the Apply for the JJurisprudence, Ethics and Professionalism Exam page.
You must submit a request in writing to [email protected]. You must also log into your Prometric account to cancel your exam appointment.
For more information on exam withdrawal deadlines, fee refunds and documentation, please see Apply for the Jurisprudence, Ethics and Professionalism Exam.
Knowledge Assessment
The Knowledge Assessment is administered by an external vendor and is only available once per year during the month of May. It cannot be completed outside of this period. You may request a one-time deferral to the following session (i.e., the following May), which may be granted on a case-by-case basis for the following reasons:
- Medical circumstances, with supporting documentation
- Parental leave (maternity, paternity, or parental), with an expected return date
- Pre-booked travel, with supporting documentation and return date
- Conflicts with academic or professional exams/courses, with supporting documentation
- Other major, unexpected, or extenuating circumstances, considered on a case-by-case basis
Any second or subsequent deferral requests must include supporting documentation and will be referred to the Quality Assurance Committee for review and decision.
To request a deferral, please email [email protected].
Participation in the Quality Assurance Program is a requirement under the Regulated Health Professions Act. Failure to participate in the Knowledge Assessment when selected will result in a referral to the Quality Assurance Committee. The Committee has the authority to direct actions that can significantly impact your ability to practice, including transferring your certificate of registration from Part A to Part B of the Register.
If you are unsuccessful in the proctored Knowledge Assessment, your results will be reviewed by a panel of the Quality Assurance Committee to provide recommendations for remediation.
If you are unsuccessful on the Knowledge Assessment, you will receive a performance profile that will help you identify your areas for improvement. You should review appropriate materials to address your knowledge gaps.
If you are unsuccessful on your first attempt of an unprotored Knowledge Assessment, you will be given a second opportunity during the same month to successfully complete the Knowledge Assessment. This reassessment attempt will also be unproctored. If you are unsuccessful on your second unproctored attempt, you will be required to complete a period of remediation and undergo a proctored Knowledge Assessment within six months.
To successfully complete the assessment, a pharmacist’s performance must meet or exceed the final pass score that is set using a criterion-referenced pass/fail standard. While similar, the pass score varies with each knowledge assessment based on the questions that are selected and reflects the overall level of difficulty of a particular Knowledge Assessment. This process helps standardize different assessments.
Pharmacists are required to complete a proctored Knowledge Assessment in any of the below situations:
- Is a Part A pharmacist and has been unsuccessful at a knowledge reassessment (i.e., has not successfully completed the Knowledge Assessment on two attempts)
- Is a Part A pharmacist and has been unsuccessful on a practice reassessment
- Is a Part B pharmacist requesting to move to Part A of the Register
At least fifteen minutes before your scheduled appointment time, log into the testing platform to ensure your technology is operational. At your appointment time, complete the check in process with the proctor. You will have up to three hours to complete the assessment which consists of 45 questions. The CPS will be accessible through a link within the assessment. Other electronic resources are permitted through a monitored browser.
If requested in advance, the proctored Knowledge Assessment can also be taken in person, with locations varying based on your geographical area.
Your result will be available immediately after the completion of your assessment. Knowledge Assessment results are not shared with employers or colleagues.
Because unproctored Knowledge Assessments are completed in an unsupervised environment, you may begin the online assessment anytime within the one-month window.
Once you start, you will have up to three hours to complete the assessment in one sitting and can reference resources of your choice. The assessment consists of 45 questions.
Your result will be available immediately after completing the assessment. Knowledge Assessment results are not shared with employers or colleagues.
Candidates must complete the Knowledge Assessment in one sitting. Although candidates are not able to “pause” the assessment or complete it over several days, short breaks are permitted and candidates have up to three hours to complete the 45-question assessment.
As the Knowledge Assessment focuses on assessing an individual’s ability to apply clinical knowledge as well as current legislation, ethics and scope of practice to patient care scenarios, the assessment must be completed independently. Additionally, all candidates are required to review and agree to a statement of understanding which includes maintaining the confidentiality of the Knowledge Assessment exam questions.
For troubleshooting support for the Knowledge Assessment testing platform, contact Meazure Learning directly. Support is available 24/7.
Real-Time Chat
Phone: 1-855-772-8678, Option 1
If you take the Knowledge Assessment outside of College hours, OCP staff will not be available to immediately assist. Any calls or emails will be addressed on the next business day. The hours of operation for the College are Monday to Friday, from 8:30 a.m. to 5 p.m.
Meazure Learning is the administrator of the Knowledge Assessment. The College is working alongside Meazure Learning to deliver the knowledge assessment on the Meazure exam platform.
Leading up to the Knowledge Assessment, you will receive important communication from Meazure Learning with login details for the Meazure exam platform and information on next steps.
All Meazure Learning emails related to your Knowledge Assessment will be sent from Testing Support ([email protected]). Please add [email protected] to your Safe Senders list to ensure you receive these important messages.
Requests for testing accommodation(s) will be considered for the exam due to a disability or impairment (temporary or permanent) that substantially limits life activities or has a direct impact on testing processes. Supporting documentation must be submitted to the College by emailing [email protected] no later than 45 days prior to the exam date (or as soon as possible). For more information, please review the College’s Guidelines for Testing Accommodations.
When you are notified that you must undergo a knowledge assessment, you should review the Knowledge Assessment blueprint that describes the key topics and competencies that will be assessed, and provides examples of topics.
Note that the best preparation is your ongoing patient care practice. Pharmacist peers have created the assessment cases to be reflective of everyday practice.
You could also review the practice resources and the current, reliable, and evidence-based references that you typically use in your practice as you will have access to these during your knowledge assessment. Additional resources include:
- OCP’s e-learning modules, including Code of Ethics and jurisprudence
- CPhA’s therapeutic information database CPS (formerly RxTx). Prior to the assessment, a link to CPS will be provided to you by the College at no cost. Candidates may use their personal CPS account or the CPS link provided by the College.
An online platform demonstration and sample questions will be made available before your assessment.
For more information on preparing for the assessment, visit our preparing for the knowledge assessment webpage.
The assessment is an online, multiple choice question, open-book exam taken remotely from a home or workplace. Registrants will have access to resources and references of their choice and will be required to apply relevant information to make patient care decisions. Prior to the assessment, a link to CPS will be provided to you by the College at no cost. Candidates may use their personal CPS account or the CPS link provided by the College. Accessing appropriate resources reflects real practice where pharmacists are expected to use reliable, evidence-based and current references to support patient care.
The College’s Quality Assurance Program is grounded in current best practice which indicates that multiple yet complementary assessment modalities employed on different occasions in the practitioner’s practice is the best approach.
With a focus on engagement and learning, the Knowledge Assessment works alongside the other elements of the Quality Assurance Program to drive enhanced patient outcomes. The four components of the Quality Assurance Program are described below:
- Knowledge assessment: focuses on knowledge and skills needed to provide appropriate care through a standardized computer-based assessment
- Practice assessment: focuses on the processes used to deliver patient care and ability to meet key standards of practice through conversation with a College practice advisor at the place of practice
- Learning portfolio (self-directed): independently engage in ongoing professional development to maintain competency and advance practice based on specific place of practice and patients
- Self-assessment (self-directed): identify learning needs to inform self-directed professional development
All Part A pharmacists are required to complete the Knowledge Assessment when selected.
Part of the College’s commitment to protecting the public is helping to ensure that pharmacy professionals maintain appropriate skills and knowledge throughout their career. The Quality Assurance Program assures the public that pharmacy professionals are practicing to the standards of the profession, and are engaged in safe and quality care.
The Knowledge Assessment is one component of the Quality Assurance Program and is distinct from other components of the Program in that it assesses pharmacists’ ability to apply clinical knowledge as well as current legislation, ethics and scope of practice to patient care scenarios. All components of the Quality Assurance program work together to support the delivery of safe, quality care.
By being part of an integrated Quality Assurance Program, the Knowledge Assessment is highly relevant to a registrant’s growth and development as a regulated pharmacy professional and to the College’s duty to assure the public that pharmacy professionals possess the knowledge and skills necessary to provide quality care.
The Knowledge Assessment is part of the multi-modal approach to quality assurance that has been adopted for pharmacists in all practice settings. The Knowledge Assessment is designed to ensure core current knowledge for those pharmacists providing patient care (pharmacists in Part A of the Register).
The purpose of the Knowledge Assessment is to encourage continuing professional development by supporting pharmacists to validate their knowledge and identify learning needs and seek appropriate education or resources to address those areas for development. The Knowledge Assessment assesses pharmacists’ ability to apply clinical knowledge as well as current legislation, ethics and scope of practice to patient care scenarios.
Grounded in the principles of quality assurance and continuous professional development, the Knowledge Assessment promotes life-long learning. This is important to helping maintain competency throughout a registrant’s career and optimize the health outcomes of the patients they serve.
Yes, the Knowledge Assessment is a required component of the College’s Quality Assurance Program for all Part A pharmacists.
A Quality Assurance Program is required in statute for all healthcare regulators in Ontario under the Regulated Health Professions Act. The purpose of the Quality Assurance Program is not only to assure the public that healthcare professionals are competent to provide patient care, but also to contribute to individual and system-wide continuous quality improvement.
All Part A pharmacists are required to complete the Knowledge Assessment when selected.
Language Proficiency
Language proficiency requirements help ensure applicants and registrants can effectively communicate with the College, patients and other healthcare professionals to support safe and quality patient care throughout their pharmacy careers. Meeting language proficiency requirements is a legal requirement under O. Reg. 256/24.
The English and French language proficiency tests and cut scores were updated to align with NAPRA’s Language Proficiency Requirement Policy. The 2024 NAPRA cut scores were determined through a rigorous scientific standard setting process with panels comprised of pharmacy practitioners, educators and regulators. The new cut scores reflect the evolution of pharmacy practice since the former scores were set in the mid-1990s and now align with other majority English-speaking countries.
The non-NAPRA language proficiency tests are ones that are approved by the Government of Canada for immigration purposes. Ontario Regulation 508/22 of the Regulated Health Professions Act, 1991 requires the College to accept those tests. The cut scores for the other tests were determined from the 2024 NAPRA cut scores using published equivalence scales, such as the Common European Framework of Reference for Languages/Cadre européen commun de référence pour les langues (CEFR/CECR) and the Canadian Language Benchmarks/Niveaux de compétence linguistique canadiens (CLB/NCLC).
If you completed your language proficiency test between January 1, 2024, and December 31, 2025, you must meet the tests and cut scores outlined on our pharmacist webpage (for pharmacist applicants) or our pharmacy technician webpage (for pharmacy technician applicants).
Test scores are valid for two years from the test date. If you do not register with the College within two years of the date of your language proficiency test with acceptable cut scores, you may be eligible to request a 1-year extension of the validity of your test. Please refer to the FAQ about expired test scores.
Yes, all components of the test must be attempted in the same session for the results to meet the College’s language proficiency requirement. The College does not accept the TOEFL MyBest Scores.
For the OET Test on Computer and the OET@Home formats, the listening, reading and writing sub-tests must be taken together and the OET speaking sub-test must be completed within the same test administration (usually eight days before or after the listening, reading and writing test).
The College can verify most test scores online. International pharmacy graduates who are required to apply through the Pharmacists’ Gateway Canada should upload their CELPIP, IELTS or TEF Canada test score reports to their Gateway account.
- For CELPIP, applicants should ensure that they have shared the test results with the College on the CELPIP portal.
- For IELTS, the Test Report Form number in the bottom right corner of the report must be legible.
- For PTE Core, applicants should ensure that they have shared their score with the College and provide their Registration ID or provide their Score Report Code (a 10-character code written at the top of the score report).
- For TEF Canada, the Attestation number in the bottom left corner of the report must be legible.
Otherwise, test score reports must be sent directly to OCP by the testing institution.
Test scores are valid for two years from the date of the test. Your test scores must be valid every time you submit an application.
When you are ready to submit your application for registration, please e-mail [email protected] if you would like to request consideration for an extension of your test scores. If you recently successfully completed a bridging program approved by the College’s Board, the Practice Assessment of Competence at Entry (PACE), Structured Practical Training (SPT), or the PEBC Qualifying Exam, a College staff member may be able to extend the validity of your scores.
If you have not successfully completed a current practice-based assessment or examination registration requirement, your request will be referred to a panel of the Registration Committee.
For more information, please see the “Extending the validity of an applicant’s language proficiency test scores” section of the Language Proficiency Requirements at Registration for All Applicants Policy.
Your supporting documentation should be submitted to [email protected] when you submit your application for registration as an intern or as an intern technician. Please include your name and OCP number in your email so that the evidence can be added to your existing OCP account.
For more information, please see the Non-Objective Evidence of Language Proficiency policy and the relevant pharmacist applicant or pharmacy technician applicant registration pathway.
There is no restriction on the type of information you may submit to a panel. However, it must be sufficiently reliable and persuasive to satisfy a panel that you possess reasonable language proficiency in English or French. The following is a list of examples you might submit:
- Evidence of previously acceptable English or French language proficiency test scores (as listed on the language proficiency requirements for pharmacists or language proficiency requirements for pharmacy technician pages)
- Evidence that your elementary, secondary and pharmacy education was completed in English or French
- Evidence that you obtained your pharmacy degree at a faculty of pharmacy where the language of instruction was English or French, followed by practice in an English or French speaking environment
- Evidence of a long-standing history of working as a pharmacist where direct patient care and collaboration with other health professionals were provided in English or French verified by regulated professionals or recognized academics
- Evidence of your having authored pharmacy publications in English or French, accompanied by evidence from your professor/supervisor attesting to your language proficiency
No. Since 2006, the College’s language proficiency requirements has included a process that allowed the College to investigate if a registrant was reported to be unable to effectively communicate in English or French. In 2024, changes to NAPRA’s Language Proficiency Requirement Policy and Ontario Regulation 256/24 – General Regulations under Pharmacy Act, 1991 prompted the College to update and more clearly define the language proficiency expectations for applicants and registrants. A declaration confirming a registrant’s ability to speak, read, write and comprehend English or French with sufficient fluency to practise the profession will be introduced as part of the 2026 annual renewal process.
No. Any concerns about a registrant’s language proficiency will be addressed through the College’s Complaints & Reports process. The first step will be gathering information to determine the validity of the concern.
Learning Portfolio
Because individual learning needs are unique and may vary each year depending on their practice, the College does not set a required number of learning activities or contact hours to be obtained each year. You are responsible for identifying your learning needs, choosing the activities to achieve your goals and applying your learnings to your practice. Emphasis should be on the quality of your learning and how it relates to your practice rather than the number of hours spent learning.
If you would like a pre-determined number of learning hours or activities to complete each year, set yourself a realistic target number at the beginning of the year and work towards that goal. For example, some other provincial pharmacy regulatory authorities have set 15 to 20 hours as the annual required minimum. Documenting each of your learning activities in the learning portfolio will help you track your progress and determine when your goals have been achieved.
You may document learning activities that involve a structured traditional format, such as attending a workshop or completing a self-study course. You may also include non-traditional self-directed learning resources, such as reading articles on a particular topic or discussing an issue with colleagues. Any learning activity that helps you to achieve your learning goal may be documented
Maintaining Your Registration
Please contact [email protected] to request a letter of standing (e.g., for registering with another regulatory body).
Minor Ailments
There is no cost for patients accessing this pharmacy service under Ministry of Health funding; patients only require their Ontario health card. If a prescription is issued to treat the minor ailment, similar to prescriptions provided by a physician or nurse practitioner, there may be costs or fees associated with dispensing.
Yes, and yes. Pharmacists may prescribe for minor ailments, regardless of the nature of their workplace. For example, pharmacists in a Family Health Team (FHT) or Nurse Practitioner Led Clinic (NPLC) can issue a prescription for a minor ailment to be dispensed at the patient’s chosen pharmacy.
However, in a hospital setting, regulations under the Public Hospitals Act do not permit pharmacists to ‘order treatment’ for inpatients or outpatients. Pharmacists practicing in hospitals may wish to explore delegation of authority to prescribe, such as a medical directive, in accordance with organizational policies.
At the time the medications associated with the minor ailments were being identified, the intent of using drug categories was to have the flexibility to prescribe the most up-to-date medications available on the market. With a list of drugs written into the regulations, adding any new drugs to the Schedule would require going through the regulatory amendment process.
Due to concerns the American Society of Health-System Pharmacists (ASHP) raised about acquiring a license to utilize the AHFS classification system, which is a proprietary product, the College submitted drug lists for all of the previous and new minor ailments as part of these amendments.
No. Pharmacists are only authorized to prescribe the specified drugs listed in Column 3. At this time, expert clinical opinion advises against prescribing high-potency topical corticosteroids and ophthalmic fluoroquinolones for their respective minor ailments, and as such, they are not listed in Column 3.
Yes. Pharmacists can only prescribe a drug in listed in Column 3 of Schedule 4 for the respective minor ailment, even if it is in a combination product. Each drug found in the combination must be listed in Schedule 4 of O. Reg. 256/24; this was taken into consideration when the proposed regulations were submitted to the Ministry.
Yes. The Standards of Practice expect pharmacists to use evidence from relevant sources to inform their activities and to critically evaluate medication and related information. Pharmacists have an ethical obligation to ensure information provided to patients is current and consistent with the best available evidence.
In pharmacies, it is a standard of accreditation to have available the references and resources pharmacists require to meet the standards of practice of the profession and to support the pharmacy services provided. Pharmacists may choose to subscribe to or use any clinical resources that will enable them to provide safe and effective patient care.
While the regulations authorizing pharmacists to prescribe for minor ailments do not include age restrictions, it is possible that characteristics such as age may be relevant to the treatment of specific minor ailments. Pharmacists should conduct a patient assessment and use their knowledge, skills and judgment along with available clinical treatment algorithms to determine whether age or any other factor might indicate that prescribing for a particular minor ailment is contraindicated. If a decision is made not to prescribe, that decision must be explained to the patient along with a follow-up plan for monitoring and/or next steps. This could include a referral to another healthcare provider.
Registered pharmacy interns have the same scope of practice as a pharmacist, subject to the supervision requirements set out in the terms, conditions, and limitations on their certificate of registration. Please refer to the Supervision of Pharmacy Personnel Policy for more information.
As with any area of practice, the supervising pharmacist must assess the registrant’s competence to provide a service and engage in patient care, and the degree of supervision required, to do so safely and effectively. In addition, the registrant is expected to complete the Mandatory Orientation for Minor Ailments (Ailment) Prescribing module to ensure they understand their ethical, legal, and professional obligations associated with this new scope.
Pharmacy technicians can gather and document relevant health information from the patient and conduct a Best Possible Medication History to inform the pharmacist’s patient assessment and support their decision-making. They can also assist the pharmacist in documenting and notifying the patient’s primary care provider about the minor ailment service and, if one is issued, the details of the prescription.
The Designated Manager should have processes in place to incorporate pharmacy technicians into the workflow for minor ailment services and to support registrants in practicing to their full scope. Pharmacy technicians cannot “provide information or education relating to drug use, either to or for a patient, where the provision of the information requires therapeutic knowledge, clinical analysis or clinical assessment” due to the terms, limitations, and conditions on their certificate of registration.
The Code of Ethics, Standards of Practice, and relevant Policies and Guidelines, including the College’s Virtual Care Policy apply when providing minor ailment services virtually. Importantly, the pharmacist must first determine that the manner in which virtual care is provided is suitable for the patient assessment and will enable them to meet all legal and professional obligations.
For the minor ailment service to be eligible for reimbursement through the Ontario Drug Benefit Program, it must be provided in-person or virtually (including by phone) from an eligible pharmacy location, as described in the Executive Officer Notice: Funding for Minor Ailment Services in Ontario Pharmacies and Questions and Answers for Pharmacies from the Ministry of Health.
As with other services that are not covered by a publicly funded program and which are over and above the services included in the usual and customary dispensing fee, pharmacists may charge fees for professional pharmacy services in accordance with the College’s Fees for Professional Pharmacy Services Policy. For example, it should be clear to the patient what the fee is for, and the charge should be reasonable for the service provided.
Patients with private insurance or health benefits may inquire with their provider regarding possible reimbursement of any fees for minor ailment services, and pharmacists are encouraged to provide an invoice or receipt upon request for patients to use for this or other purposes.
Having the legislated authority to prescribe for minor ailments does not mean all pharmacists must provide this service. Patients are encouraged to speak to their pharmacist to find out what healthcare services they offer.
Pharmacists have an ethical obligation to ensure that they only practice when they are competent to do so, as self-assessed with respect to both relevant knowledge and skill as well as physical, emotional and mental capacity. The practice environment must also meet the Standards of Operation and be conducive to the safe and appropriate provision of pharmacy services. As such, pharmacists have the autonomy to incorporate minor ailments prescribing into their practice as they see appropriate, which may include a staggered implementation of minor ailment prescribing, or offering services for some, but not all, minor ailments. When unable to provide a requested pharmacy service, the pharmacist assumes responsibility for making reasonable efforts to ensure continuity of patient care, such as a referral to another pharmacist or practitioner.
Yes. The regulations and College guidelines do not specifically address or prohibit refills. However, it is important to remember that a minor ailment is usually a short-term condition where only minimal or short-term follow-up is required, and that if the prescribed treatment is not effective, further assessment and/or a referral to another health care professional may be warranted.
The pharmacist should use their professional judgment to determine whether issuing refills at the time of prescribing is clinically appropriate for the indication and document their rationale. For example, the amount of topical cream the patient might need for their expected duration of treatment can be approximated, but not necessarily exactly calculated, and the pharmacist may decide prescribing an initial quantity with a refill is appropriate. The follow-up and monitoring plan should ensure that the intended timeframe of the patient’s therapy is adhered to. A pharmacist dispensing a refill for a minor ailment must evaluate the request in accordance with the Standards of Practice, taking into account the ongoing need considering the minor ailment and an assessment of the patient.
If a patient chooses to have a lesser amount than the prescribed quantity dispensed, they should be advised if additional dispensing fees will be charged and the patient must authorize this request in writing (as per the Drug Interchangeability and Dispensing Fee Act [DIDFA]).
The regulations require a pharmacist to notify “the patient’s primary care provider (if any) within a reasonable time” that they prescribed a drug for the patient. If there is no primary care provider to notify at this time, this information is retained on the patient record in accordance with legislation and must continue to be accessible to the patient and their circle of care.
As the prescriber, the pharmacist is responsible for monitoring and follow-up on the treatment plan and prescribed therapy, essentially assuming the role of primary care provider until the patient’s care can be transitioned, if necessary, to another healthcare professional.
Minor Ailments Prescribing Module
The mandatory Orientation for Minor Ailments Prescribing module is presented in an online browser-based format that may be accessed by any desktop computer, tablet, or smartphone. The module is narrated and will require audio capability; however a transcript of the narration is also available in the module’s menu tab. Quizzes to assess knowledge are embedded within the module though scores are not recorded and pharmacists do not need to achieve a particular grade to pass or complete the orientation.
It should take approximately one hour to complete, and it does not have to be completed in a single session. Users may continue their progress at any time.
To support understanding of the scope of practice change authorizing pharmacists to prescribe for minor ailments, all Part A pharmacists must complete the mandatory Orientation for Minor Ailments Prescribing module about the legislative requirements regardless of their practice setting or whether they choose to prescribe for minor ailments. This decision was made the Board in June 2020, based on the fact that the regulation change applies to the profession as a whole and not to a specific area of practice.
The mandatory Orientation for Minor Ailments Prescribing module is designed to ensure all Part A pharmacists fully understand their ethical, legal, and professional obligations when prescribing for minor ailments, and available free of charge on the College’s website. The content is not clinical in nature, although optional continuing education courses are available for registrants.
No, the mandatory Orientation for Minor Ailments Prescribing module is available free of charge on the College’s website.
The mandatory Orientation for Minor Ailments Prescribing module should take approximately one hour to complete, and it does not have to be completed in a single session. Users may continue their progress at any time.
It is the expectation of the College that pharmacists possess the required clinical skills, judgment and knowledge of legislative requirements and practice standards to safely prescribe medications for minor ailments. To refresh their clinical knowledge and increase their comfort in assessing and prescribing for minor ailments, pharmacists may consider additional education opportunities. A list of online continuing education courses related to minor ailments are available on the College’s website. These clinical education courses are optional, and pharmacists will not be expected to attest to their completion.
No. Following the initial implementation of prescribing for minor ailments in 2023, the College considers prescribing for minor ailments as part of usual practice and has moved to incorporate this expanded scope into its entry to practice, operations and quality assurance assessments. For pharmacists who were licensed after December 31, 2023, the College’s expectation is that the information covered in the College’s Orientation for Minor Ailments Prescribing module has been covered in curriculum and as part of entry to practice preparation.
Therefore, you are not required to inform the College that you have completed the Orientation for Minor Ailments Prescribing module. However, the College expects that, prior to prescribing for minor ailments, you understand your ethical, legal, and professional obligations described in the module and that you possess the required knowledge, clinical skills, and judgment to safely assess and prescribe medications for minor ailments.
Pharmacy interns have the same scope of practice as a pharmacist, subject to the supervision requirements set out in the terms, conditions, and limitations on their certificate of registration. The mandatory orientation module is designed to ensure registrants fully understand their ethical and legal requirements of prescribing for minor ailments prior to engaging in this practice. It is the expectation of the College that they also have the necessary knowledge, skills and judgment to safely deliver this service when providing patient care.
To support understanding of this scope of practice change, all Part A pharmacists must complete the mandatory Orientation for Minor Ailments Prescribing module about their ethical, legal, and professional obligations before prescribing for minor ailments. Completion is required regardless of a pharmacist’s practice setting or whether they choose to prescribe for minor ailments. This decision was made by the Board in June 2020, based on the fact that the regulation change applies to the profession as a whole rather than to a specific to area of practice.
Non-Sterile Compounding
Yes, this course is free of charge and open to anyone. Anyone who wishes to supplement or refresh their training is encouraged to take the course (e.g., moving to a new pharmacy with a different level of requirements in place, returning to the non-sterile compounding supervisor role after an extended leave).
Registrants who choose to complete this training course voluntarily are not required to self-declare in the OCP Portal and can retain their certificate of completion for their own records.
New non-sterile compounding supervisors will need to sign in to the OCP Portal and confirm they have completed the mandatory training under the Authorized Training tab.
The final exam must be taken to generate a certificate of completion. Non-sterile compounding supervisors should retain a copy of their certificate for their records and ensure it is available to the College upon request, for auditing purposes. The College will collect the documentation it needs to verify the requirements have been met when it is appropriate to do so.
College operations advisors will follow-up with non-sterile compounding supervisors whose pharmacies have not met the standards after their pharmacy assessments to confirm completion of the training course.
New compounding supervisors will need to self-declare they have completed the mandatory training course. (See FAQ: “I am starting a new role as a compounding supervisor. How do I self-declare that I have completed the training?”)
New non-sterile compounding supervisors are required to complete the training course within three months of starting the role, although it is preferred that they complete the training course before starting.
Current non-sterile compounding supervisors whose pharmacies are not meeting the standards will have one month to complete the training course; however, College operations advisors may extend this period as appropriate to the circumstances.
The final exam must be taken in order to confirm completion of this mandatory training course.
The mandatory training goes into effect immediately: February 5, 2026.
Current non-sterile compounding supervisors in community and hospital pharmacies are required to complete the training course to address identified gaps if an operational assessment reveals that a pharmacy does not fully meet the standards. In this event, the College operations advisor will assign the training course as part of the action plan in the pharmacy’s assessment report.
The non-sterile compounding supervisor training course was designed to be taken in its entirety; however, there may be circumstances in which College operations advisors recommend or require completion of select modules from the training course. These will be handled on a case-by-case basis.
A “new” non-sterile compounding supervisor is defined as someone who is new to the role. Any pharmacist or pharmacy technician who is assigned as a non-sterile compounding supervisor on or after February 5, 2026 is responsible for taking this mandatory training course. If you became a new compounding supervisor before this date, this requirement does not apply. However, we strongly encourage all registrants in a non-sterile compounding supervisor role take the training course to meet any self-identified learning needs and goals (e.g., if you feel like you need to reinforce your existing training, are coming back from an extended leave, or are taking this position in a new pharmacy with a higher level of requirements in place).
In a community pharmacy, the Designated Manager (DM) is the non-sterile compounding supervisor unless they have assigned this role to another pharmacist or a pharmacy technician.
In a hospital setting, the pharmacy manager/department head of the hospital pharmacy is the non-sterile compounding supervisor, unless they have assigned this role to a pharmacist or pharmacy technician.
At the March 2024 Board meeting, the Board approved mandatory training for new compounding supervisors in all pharmacies and current compounding supervisors in pharmacies where standards are not being met. The decision to require OCP-approved training was in response to operational assessment data showing standards are not being fully met, in part due to insufficient training of compounding supervisors. This decision follows the principles of right-touch regulation in which a regulatory response is required if the risk of harm to patients is high.
Health Canada considers compounding to be the following:
“The combining or mixing together of two or more ingredients (of which at least one is a drug or pharmacologically active component) to create a final product in an appropriate form for dosing. It can involve raw materials or the alteration of the form and strength of commercially available products. It can include reformulation to allow for a novel drug delivery. Compounding does not include mixing, reconstituting, or any other manipulation that is performed in accordance with the directions for use on an approved drug’s labelling material.”
Before compounding a non-sterile preparation, the need for the compounded product should be confirmed by checking for commercially available preparations in the Health Canada’s Drug Product Database and contacting manufacturers. To comply with the Health Canada policy on compounding, this confirmation is required in order to validate the lack of product availability and avoid duplicating an approved drug.
Non-sterile preparations can be categorized as simple, moderate or complex (as outlined in United States Pharmacopeia (USP) Chapter <795> Pharmaceutical Compounding – Nonsterile Preparations). A number of factors go into determining the type of preparation and level of risk when compounding preparations. Pharmacists and pharmacy technicians who compound non-sterile preparations should evaluate their practice, develop service-related procedures and implement appropriate quality controls for both patients and compounding personnel, with a view to guaranteeing the overall quality and safety of non-sterile preparations.
The National Institute for Occupational Safety and Health (NIOSH) website explains that a hierarchy of controls is way to identify a preferred order of actions to best control hazardous workplace exposures. Compounding supervisors should review the descriptions of each level of control. The diagram below* provides examples of what the various controls might be feasible in the context of pharmacy compounding to minimize risk of exposure to occupational hazards.
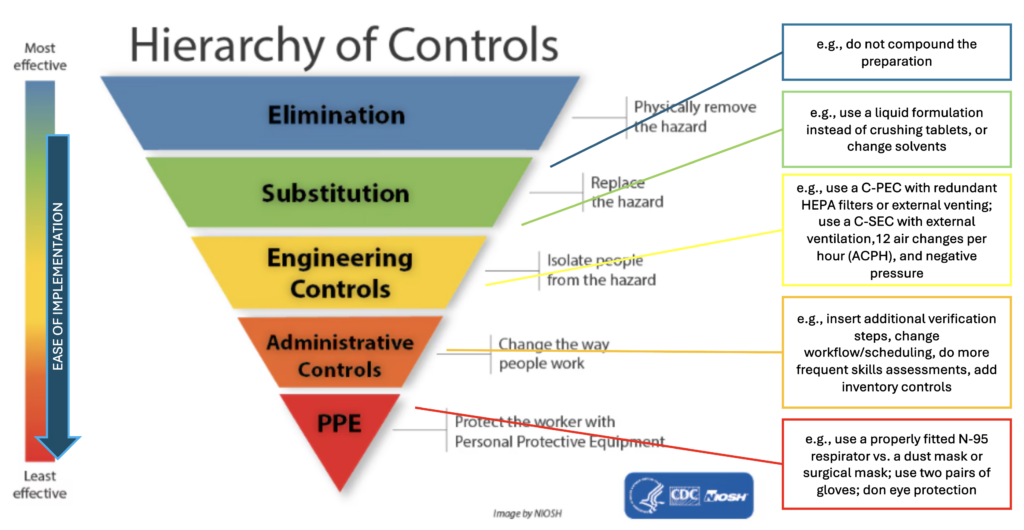
*Adapted from: https://www.cdc.gov/niosh/topics/hierarchy/default.html.
It is the responsibility of the compounding supervisor and manager to evaluate and choose the appropriate controls, depending on the type of risk posed by a hazardous product. Key points to consider in your decision-making are:
- The level of risk the ingredient(s) may present
- The volume/frequency of each ingredient used
- The combined exposure to all higher risk ingredients
Each prescription and patient situation must be assessed individually. In the event it is necessary to compound a particular preparation requiring processes and safety measures that are not currently in place in the pharmacy, the documentation should specify the following:
- Potential risks of compounding the preparation without the processes and safety measures currently in place
- The extra steps that must be taken to mitigate these risks
- References confirming that these steps will actually minimize risks to the quality of the product and safety of personnel
Procedures for mitigating risk must be documented in the master formulation record and reviewed at least every 12 months.
Refer to sections 9, 9.1.1, 9.2.1, 9.2.3 and 9.6 of the NAPRA Guidance Document for Pharmacy Compounding of Non-Sterile Preparations and the Pharmacy Connection article — A Closer Look at Personal Protective Equipment. Note that where the guidance document uses the language of “should,” it may be acceptable to meet the required standard using other technologies, techniques, materials and procedures than those suggested only if they have been proven to be equivalent or superior to those described in the guidance document.
The NAPRA Non-Sterile Compounding Standards do not assign a specific numerical value to define “small quantity” or “occasional.”
What constitutes an “occasional small quantity” for a preparation or ingredient depends on the amount handled and how frequently it is used; however, these cannot be considered in isolation.
First, amounts and frequency are not the only factors involved in assessing the overall degree of risk to pharmacy staff and patients (and subsequently the appropriate level of requirements [A, B or C] for a preparation that contains a hazardous product).
Second, the cumulative risk of exposure over time must also be considered, even if the preparations are compounded on different days. The level of requirements must be sufficient for the pharmacy’s compounding practice as a whole to minimize contamination of compounded products and to provide adequate protection for compounding personnel, other staff and patients entering the premises.
For example, if a pharmacy compounds many different products “occasionally,” it is possible for their level of requirements to be the same as that of a pharmacy that compounds a few products “routinely.” When it doubt, err on the side of caution. If there is uncertainty about which risk level to assign, go with the higher standard – quality patient care and safety for all personnel should always be the top priority.
The Designated Manager (DM), pharmacy department head or non-sterile compounding supervisor (if one has been appointed) is responsible for determining whether an amount is “small” or “large,” or a frequency is “occasional” or “routine.” Conducting a risk assessment for each compounded preparation will help you determine the most appropriate level of risk, while documenting these risk assessments can help you support your rationale during a pharmacy operational assessment.
Refer to Section 4.1 and 4.2, Section 9 of the NAPRA Guidance Document for Pharmacy Compounding of Non-Sterile Preparations. Note that where the guidance document uses the language of “should,” it may be acceptable to meet the required standard using other technologies, techniques, materials and procedures than those suggested only if they have been proven to be equivalent or superior to those described in the guidance document.
Schedule 2 of the Hazardous Products Act (HPA) lists the health hazard classes, not individual products. The health hazard(s) for an active pharmaceutical ingredient (API) are identified on its safety data sheet under Section 2 – Hazards Identification.
The health hazard classes can be identified by a pictogram (see an example below), which also appears on the API’s label.
Safety data sheets provide information about a product’s risks and include recommended preventive measures to protect staff, such as engineering controls and personal protective equipment (Section 8). This is helpful in identifying products that are volatile, aromatic, sensitizing or very irritating to the respiratory tract, skin or mucous membranes as part of the risk assessment and determining the level of requirements needed.
The information in the safety data sheet must be interpreted and applied in the context of the pharmacy’s compounding practice. Compounding supervisors must consider the potential exposure of both staff and patients to the product (e.g., the physical and characteristics and chemical properties of the API, the frequency and duration of manipulation, and the potential for contact).
Note: Safety data sheets are available from the API supplier or through the Canadian Centre for Occupational Health and Safety and are updated every three years. They should be readily accessible and retrievable, and kept in a location known to all pharmacy personnel.
Manufactured drugs approved by Health Canada (i.e., those with a drug identification number [DIN]) are exempt from the HPA and therefore do not have safety data sheets. If a Health Canada–approved drug is used in a preparation, the safety data sheet for the API(s) in that drug should be consulted. The drug’s product monograph should also be considered, as it includes information on storage, stability and disposal and special handling instructions
If a compound is made up of more than one API or drug, then all of the available safety data sheets and product monographs (if applicable) must be consulted to complete your risk assessment.
Refer to Section 4.3, 6.3.4, 8, 8.1, 8.2, 8.3 and Glossary of the NAPRA Guidance Document for Pharmacy Compounding of Non-Sterile Preparations. Note that where the guidance document uses the language of “should,” it may be acceptable to meet the required standard using other technologies, techniques, materials and procedures than those suggested only if they have been proven to be equivalent or superior to those described in the guidance document.
The National Institute for Occupational Safety and Health (NIOSH) is a recognized occupational health and safety agency, and part of the U.S. Centers for Disease Control and Prevention. The NIOSH List of Hazardous Drugs in Healthcare Settings, 2024 places hazardous drugs into tables based on specific criteria.
Any drug or active pharmaceutical ingredient (API) that is not included on the NIOSH list did not meet their specific criteria for a hazardous drug. However, it is important to read the NIOSH document in its entirety (i.e., do not only refer to the list of drugs in each table). The actual risk to personnel depends on how the drug is handled and what risk mitigation measures are in place.*
The Hazardous Products Act (HPA) is the legislation that that enforces safety measures. It establishes hazard classifications and mandates that any supplier who sells or imports a hazardous product intended for use, handling or storage must provide cautionary labelling requirements and a safety data sheet (SDS).
The Workplace Hazardous Materials Information System (WHMIS) is Canada’s hazard communication standard to support the safe use of hazardous materials in the workplace. It includes products used in various workplaces – a far wider group of chemicals than NIOSH. These are categorized as either a physical hazard or a health hazard (see Schedule 2 of the HPA). It also identifies products that can be very irritating to the respiratory tract, skin or mucous membranes, which is important for determining the level of requirements.
Both NIOSH and WHMIS provide information on toxicities of drugs. The WHMIS also includes information on other workplace products. Both references should be consulted in the risk assessment process, as described in Section 4 of the guidance document and illustrated in the decision algorithm.
Safety data sheets and other applicable references should be reviewed, accessible and easily retrievable for every single ingredient in a compounded preparation (see next question).
Risk assessments should be done for each compounded preparation. They should be made available to all staff so they can review them and understand the risks.
*The NIOSH List is designed to help employers to identify which drugs handled by employees are considered by NIOSH to be hazardous drugs. Because new drugs and new formulations are continuously brought to market between NIOSH’s periodic updates, hazardous drug evaluation should be a continual process. Employers should establish their own procedures to identify and evaluate new drugs as they enter their workplace and, when appropriate, reassess their presence on hazardous drug lists as toxicological data become available to support re-categorization. (Source)
Refer to Sections 4.3 and 8.3 of the NAPRA Guidance Document for Pharmacy Compounding of Non-Sterile Preparations. Note that where the guidance document uses the language of “should,” it may be acceptable to meet the required standard using other technologies, techniques, materials and procedures than those suggested only if they have been proven to be equivalent or superior to those described in the guidance document.
Ventilation (the “V” in HVAC) is an engineering control intended to remove or control contaminants released in indoor work environments by bringing in fresh air. The Canadian Centre for Occupational Health and Safety (CCOHS) Fact Sheet on Industrial Ventilation may be helpful for background information on this subject, such as the purpose of a ventilation system.
For non-sterile compounding, the Designated Manager, pharmacy department head or non-sterile compounding supervisor (if one has been appointed) must determine what ventilation is required for their pharmacy practice; they can do so through their risk assessment, taking into account cumulative risk.
Ventilation must be controlled in such a way as to avoid decomposition and contamination of chemicals while maintaining the quality and efficacy of stored products and ensuring the safety and comfort of compounding personnel. For example:
- Air vents should not be located directly over work areas, to avoid contamination of the products.
- Fans are unsuitable for ventilation as they merely blow the contaminant around the work area without effectively removing or controlling them.
- Opening a window or door might introduce outdoor air, but it is uncontrollable and risks bringing in additional contaminants and disrupting the compounding environment.
The external ventilation requirements differ between Levels A, B and C and are summarized below. Refer to the guidance document additional details. Note that “external” means the air must go outside the building (not outside the room).
| Level | External Ventilation | Guidance Document Section |
|---|---|---|
| All levels | The OCP’s Standards of Operation for Pharmacies require pharmacies to be designed, constructed and maintained to ensure the integrity and the safe and appropriate storage of all drugs and medications. This includes proper conditions of sanitation, temperature, light, humidity, ventilation, segregation and security. | n/a |
| A | External ventilation is not required for the designated compounding area Refer to the requirements for all levels of compounding | 5.4.1.3 |
| B | External ventilation is not required for the compounding room. However, this dedicated room must be entirely closed off (i.e., separate from the rest of the pharmacy) and well-ventilated If a ventilated containment device (C-PEC*) is installed in the room, the C-PEC should either be externally vented (the preferred option) or have redundant HEPA filters in series. | 8.2 9.1 9.2.1 |
| C | The room in which the C-PEC is placed (the C-SEC†) must be separate, under negative pressure, and have appropriate air exchange, with external ventilation through HEPA filtration. The C-PEC installed in the C-SEC should be externally vented (the preferred option) or have redundant HEPA filters in series. | 8.3 9.1.1 9.1.2 9.1.5 9.2.1 |
Compounding personnel should also understand how the secondary ventilation system operates (i.e., the HVAC of the C-PEC) and be able to recognize and address any problems with it.
*C-PEC – containment primary engineering control, designed to minimize the exposure of workers and the environment to hazardous products when such products are being handled directly.
†C-SEC – containment secondary engineering control (i.e., room in which the C-PEC is placed).
Refer to the Sections of the NAPRA Guidance Document for Pharmacy Compounding of Non-Sterile Preparations referenced in the table below. Note that where the guidance document uses the language of “should,” it may be acceptable to meet the required standard using other technologies, techniques, materials and procedures than those suggested only if they have been proven to be equivalent or superior to those described in the guidance document.
For hazardous non-sterile compounding, the level of requirements needed (i.e., B or C) depends more on the risk(s) posed by the hazardous product than on the complexity of the preparation.
In addition to the requirements for non-sterile compounding, such as adequate space and sufficient lighting (guidance document, section 5.4), hazardous non-sterile preparations should be compounded in a separate, dedicated room. For example, compounding hazardous products containing carcinogenic drugs listed in NIOSH† Table 1, or those categorized by WHMIS‡ as a health hazard because they are very irritating to the respiratory tract, skin and/or mucous membranes, should take place in a Level C room. This means a physically separate, closed-off room with external venting through HEPA filtration, appropriate air exchange and negative pressure to avoid contaminating the environment and to further protect personnel. There should also be an appropriate containment device (C-PEC) for materials being compounded.
Because of the difficulty of removing hazardous product contamination, the surfaces of ceilings, walls, floors, fixtures, shelving, counters and cabinets in the non-sterile compounding area should be smooth, impermeable, free from cracks and crevices and made of non-shedding material.
If a separate area is not possible and non-hazardous non-sterile preparations must be compounded in the same room as hazardous non-sterile preparations, at an absolute minimum there should be policies and procedures in place to ensure that the area is meticulously cleaned to prevention any risk of cross-contamination.
If you intend on compounding both non-hazardous and hazardous non-sterile preparations, refer to the FAQ “Do we need two separate designated compounding areas for hazardous and non-hazardous non-sterile preparations?” for additional considerations.
†National Institute for Occupational Safety and Health.
‡Workplace Hazardous Materials Information System.
Refer to Sections 9.1, 9.1.1, 9.2.1, 9.2.2 and 9.3 of the NAPRA Guidance Document for Pharmacy Compounding of Non-Sterile Preparations. Note that where the guidance document uses the language of “should,” it may be acceptable to meet the required standard using other technologies, techniques, materials and procedures than those suggested only if they have been proven to be equivalent or superior to those described in the guidance document.
It is strongly recommended that the pharmacy has one set of dedicated equipment (including personal protective equipment) for compounding hazardous drugs and another set for non-hazardous drugs. Alternatively, disposable equipment should be used, if possible, to reduce the risk of cross-contamination.
If it is not possible to have dedicated equipment for hazardous compounding, at an absolute minimum there should be policies and procedures in place to ensure that equipment is meticulously deactivated, decontaminated and cleaned immediately after compounding preparations with hazardous materials, before being used for non-hazardous preparations. The pharmacy should review section 9 of the guidance document, which further outlines the requirements for hazardous preparations.
For occasional non-sterile hazardous compounding, a C-PEC* used for sterile hazardous compounding (e.g., Class II BSC or CACI†) may be used, provided it is decontaminated, cleaned and disinfected before compounding the non-sterile preparation and again before resuming sterile compounding in that C-PEC due to the higher risk posed to patients.
*C-PEC (containment primary engineering control): a ventilated device designed to minimize exposure of personnel and the environment to hazardous products when such products are being handled directly (guidance document, section 9.2). Refer to question below regarding facilities.
†BSC – biological safety cabinet; CACI – compounding aseptic containment isolator.
Refer to Sections 9.2.1, 9.2.2, 9.2.3 and 9.3 of the NAPRA Guidance Document for Pharmacy Compounding of Non-Sterile Preparations. Note that where the guidance document uses the language of “should,” it may be acceptable to meet the required standard using other technologies, techniques, materials and procedures than those suggested only if they have been proven to be equivalent or superior to those described in the guidance document.
If there is uncertainty regarding the risk level to assign a compounded preparation after completing your risk assessment, defer to the higher standard in the interest of safety. The Designated Manager/non-sterile compounding supervisor’s rationale to support the pharmacy’s decision must be well-researched and documented in the risk assessment, with supporting references.
Refer to Section 4 of the NAPRA Guidance Document for Pharmacy Compounding of Non-Sterile Preparations. Note that where the guidance document uses the language of “should,” it may be acceptable to meet the required standard using other technologies, techniques, materials and procedures than those suggested only if they have been proven to be equivalent or superior to those described in the guidance document.
No, the NAPRA Model Standards for Pharmacy Compounding of Non-Sterile Preparations (elsewhere referred to as “the standards”), adopted by the College, do not assign specific drugs, ingredients or formulations to each level of requirement (A, B or C). This is because compounding, like other aspects of pharmacy practice, is highly varied and dynamic.
For instance, risk assessments of similar formulations may yield different results across pharmacies because of the following:
- Numerous factors involved in assessing risk (e.g., quantity of ingredients being handled, frequency of compounding high-risk or low-risk preparations)
- Differences in pharmacy size, layout, personnel, number and volume of preparations compounded in the pharmacy, etc.
- Customized nature of compounded preparations, which may be altered to suit a specific patient’s needs
- Various ways risk may be mitigated (e.g., consider the NIOSH “hierarchy of controls”)
How a pharmacy chooses to conduct and document the rationale for their risk assessments so that it is reflective of their pharmacy is up to the discretion of the non-sterile compounding supervisor. For further support, the College has developed an optional risk assessment template, companion guide and examples for ensuring a risk assessment is performed for each compounded preparation.
Refer to Section 4.1 of the NAPRA Guidance Document for Pharmacy Compounding of Non-Sterile Preparations for additional factors to consider that can have an impact on the outcome of your risk assessment. Note that where the guidance document uses the language of “should,” it may be acceptable to meet the required standard using other technologies, techniques, materials and procedures than those suggested only if they have been proven to be equivalent or superior to those described in the guidance document.
UPDATED:The College has developed a risk assessment template, along with a companion guide and two examples (1% hydrocortisone powder in 1% clotrimazole cream and progesterone 400 mg suppositories) to help registrants understand what to document, reference, and consider when conducting a risk assessment. Use of the OCP template is optional, as how a pharmacy chooses to document risk assessments is at the discretion of the non-sterile compounding supervisor.
Every pharmacy that offers non-sterile compounding services must meet or exceed the minimum requirements set out in the NAPRA Model Standards for Pharmacy Compounding of Non-Sterile Preparations (elsewhere referred to as “the standards”).
The steps for conducting a risk assessment are described in Section 4 of the standards. In brief, there are three levels of requirements (A, B, and C), defined in section 8 (page 12) of the standards. These correlate to the compounded preparations’ associated risk and their complexity. The level of requirements the pharmacy needs to have in place depends on which non-sterile preparations are compounded, their complexity, how frequently they are compounded, at what concentration and in what amounts. Cumulative risk (of all compounded preparations) must be taken into account. Both risk to the preparation and risk to personnel must be considered and mitigated by adequate protection measures.
A risk assessment must be completed for each compounded preparation, taking into account every ingredient (e.g., drug, active pharmaceutical ingredient [API]) used.
A risk assessment should be conducted with appropriate resources. Safety data sheets and other applicable references should be consulted. The rationale for compounding and any risk mitigation strategies should be documented. Policies and procedures that ensure safety for personnel must be in place and documented as well.
If there is uncertainty on the risk level to assign, go with the higher standard. Quality patient care and safety of all personnel should always be the top priority.
Refer to Sections 4 and 8 of the NAPRA Guidance Document for Pharmacy Compounding of Non-Sterile Preparations. Note that where the guidance document uses the language of “should,” it may be acceptable to meet the required standard using other technologies, techniques, materials and procedures than those suggested only if they have been proven to be equivalent or superior to those described in the guidance document.
Refer to Sections 9.1, 9.1.1, 9.2.1, 9.2.2 and 9.3 of the NAPRA Guidance Document for Pharmacy Compounding of Non-sterile Preparations.
It is preferable to have separate areas for compounding hazardous and non-hazardous non-sterile preparations, however, if this is impossible and the same area is used, compounding and/or cleaning personnel must, at minimum, be assured that the area and any reusable equipment has been meticulously deactivated, decontaminated and cleaned to prevent any risk of cross-contamination from the hazardous materials before other preparations are compounded.
Because of the difficulty of removing hazardous product contamination, the surfaces of ceilings, walls, floors, fixtures, shelving, counters and cabinets in the non-sterile compounding area should be smooth, impermeable, free from cracks and crevices, and made of non-shedding material. It is strongly recommended that equipment be dedicated for compounding each of hazardous and non-hazardous drugs. Alternatively, disposable equipment should be used, if possible, to reduce the chances of cross-contamination.
The Designated Manager/department head and/or non-sterile compounding supervisor must have policies and procedure in place for the deactivation, decontamination and cleaning required after compounding hazardous non-sterile preparations. As part of the pharmacy’s quality assurance program, personnel must be trained and their work routinely assessed to ensure compliance with procedures.
Refer to Sections 5.4.1, 9.1.1 and 9.2.1 of the NAPRA Guidance Document for Pharmacy Compounding of Non-sterile Preparations.
It is a standard of accreditation for all pharmacies to have two sinks (or one double sink) in the dispensary. Accredited pharmacies engaged in non-sterile compounding must also meet the NAPRA Non-Sterile Compounding Standards to have a clean water supply, with hot and cold running water, available in or close to the compounding area.
Please refer to the table below which summarizes the accompanying Guidance to meet the Standards.
Compounding must take place within the accredited pharmacy area. Any material changes to the existing accredited area or layout require a Notice of Renovation to be submitted to the College, and an Operations Advisor will be assigned to review the proposed floor plan.
| LEVEL | Sink Location | GD Section | Comments |
|---|---|---|---|
| A | In or close to the compounding area | 5.4.1.4 | If the designated compounding area is in the dispensary, the minimum sink requirement for accreditation may serve to meet both sets of standards. Consider accessibility (for example, compounding volume and number of personnel compared to the available time and space), ease of use, potential for splashing nearby areas, etc. |
| B | In the compounding room | 5.4.1.4 | If there is a C-PEC, consider Section 9.1.1 and consult supplier regarding proper installation and certification |
| C | In the compounding room Water sources and drains should be located at least 1 meter away from the C-PEC. | 5.4.1.4 9.11 | |
| All levels | Clean, potable, hot and cold running water for washing hands and equipment Preferably made of stainless steel and having touchless control | 5.4.1.4 | Plumbing system should be free of defects that could contribute to contamination |
Refer to Section 5.2, Table 1, Table 2, Checklist 1 and Table 8.4 of the NAPRA Guidance Document for Pharmacy Compounding of Non-sterile Preparations.
There is no requirement in the College-adopted NAPRA Non-Sterile Compounding Standards for personnel to complete a formal, accredited or third-party training program. The pharmacy manager/department head (or designated non-sterile compounding supervisor) may choose to develop their own training tools and assessment program to suit their specific needs. The intended outcome is that the expertise of personnel responsible for compounding must be commensurate with their assigned duties.
The potential need for training and routine assessment is not limited to the compounding processes or technique; personnel must also be educated on policies and procedures, such as those related to attire, personal protective equipment, cleaning, and conduct.
Refer to Table 8.4 of the Guidance Document for an overview of training topics for each Level (A, B or C), as well as the specific sections relevant to the type of personnel or preparation.
- Checklist 1: Skills assessment checklist for compounding process
- Table 1: Elements to cover in training of compounding personnel
- Table 2: Elements to cover in training of cleaning personnel
- Table 3: Examples of policies and procedures
For registrants interested in exploring external courses, the College provides a listing of continuing education resources. Please note the listings are not exhaustive, and inclusion of a course is not to be construed as an endorsement.
Refer to Section 5.4 and 5.4.1.1 of the NAPRA Guidance Document for Pharmacy Compounding of Non-sterile Preparations.
The College-adopted NAPRA Non-Sterile Compounding Standards do not specify a minimum size or dimensions for Level A compounding. There must be a separate space designated for compounding with an area large enough for compounding personnel to work comfortably and safely, with room to store equipment and products in an orderly manner in clean and secure surroundings. Also, the area should be designed and arranged to prevent cross-contamination between products, and it should be located away from parts of the pharmacy where there is a considerable amount of traffic (e.g., aisles, entrance and exit).
Refer to Sections 1, 2 the NAPRA Guidance Document for Pharmacy Compounding of Non-sterile Preparations.
The College adopted NAPRA Model Standards for Pharmacy Compounding of Non-Sterile Preparations and Guidance Document for Pharmacy Compounding of Non-sterile Preparations, as the standard for non-sterile compounding in Ontario.
Standards outline the minimum mandatory expectations that must be met by the profession, using the language of “must.” Therefore, these Model Non-Sterile Compounding Standards represent the minimum requirements to be applied in compounding to ensure the overall quality and safety of non-sterile preparations.
Each standard has a corresponding section in the Guidance Document with details concerning how the standard can be achieved. The Guidance Document was developed by NAPRA as a supplemental resource and establishes professionally-accepted means by which pharmacies can achieve compliance with the standards.
Where the guidance uses the language of “should”, it may be acceptable to meet the required standard using other technologies, techniques, materials and procedures than those suggested, if they have been proven to be equivalent or superior to those described in the Guidance Document.
Refer to Sections 3 and 5 of the NAPRA Guidance Document for Pharmacy Compounding of Non-sterile Preparations.
Compounding is within the scope of practice and authorized acts for pharmacy professionals defined in the Pharmacy Act, and compounding non-sterile preparations according to recognized guidelines and standards is an entry-to-practice competency for both pharmacists and pharmacy technicians. Therefore, it is reasonable that the public and other health practitioners expect any pharmacy to provide some compounding services.
The requirements for “all levels of compounding” (Section 5), corresponding to Level A, should be attainable for all pharmacies already engaged in compounding. Moreover, the College‘s Code of Ethics expects that “registrants make every reasonable effort to provide quality cost-effective pharmacy care and services to patients and society.”
As explained in Section 3 of NAPRA’s Guidance Document, “given that pharmacists and pharmacy technicians are expected to maintain competency in basic compounding skills, they are also expected to provide compounded preparations within their level of expertise and within the limitations of available and appropriate facilities and equipment.”
As for all prescriptions, it is expected that a pharmacist will assess the prescription for a non-sterile preparation to determine whether it is the most appropriate therapy for the particular patient or if there is a suitable, safer alternative. If a compounded preparation is the best option, the Designated Manager/department head must ensure that the pharmacy has the resources necessary to safely and accurately prepare a quality preparation. If not, they have an ethical obligation to “assume responsibility for making reasonable efforts to ensure continuity of patient care when they are unable to provide requested pharmacy services.”
Ontario Pharmacy Health Program
The identity of registrants who contact OPHP directly (i.e., make a self-referral) is not shared with the College and participation in OPHP does not mean that you are no longer able to practice pharmacy.
In some cases, and upon review with your healthcare providers and the program’s medical advisor, OPHP staff may recommend that you temporarily stop practicing, allowing you to achieve medical stability and to put into place the supports you need to practice safely.
OPHP will work with you, your treatment providers and your employer to develop a plan for modified work or return to work as necessary.
Accessing OPHP is free, including providing resources, case management services, monitoring and assistance with staying at work/returning to work.
However, any medical and clinical assessments, laboratory screens and treatment (e.g., counselling) may have a cost associated with them. More information will be provided by OPHP during their intake process.
As the administrator of the program, Lifemark Health has assured the College that they are committed to privacy protection and adhere to professional standards of confidentiality at all times in compliance with the Personal Health Information Protection Act, 2004 (PHIPA) and Lifemark Health Privacy Policy.
When you contact OPHP, they can provide more details about their privacy policies should you wish to know more prior to identifying yourself.
No. The identity of registrants who access OPHP directly and the details of their conditions, are not shared with the College.
Occasionally, registrants may enroll with OPHP as part of a College proceeding or investigation, such as a health inquiry, which is an established process for when the Registrar believes a registrant may be incapacitated. In such circumstances, information would only be shared with the College by OPHP with the consent of the registrant.
You should expect:
- an evidence-based program that is specifically designed for pharmacy professionals and has been modelled from similar health programs for nurses and physicians
- support of an interdisciplinary team that specializes in mental health and addiction services
- identification of programs/services, in partnership with your current treatment team, that may address gaps in your current care/supports and strengthen your overall stability and well-being
- structured and ongoing check ins to ensure that you remain supported in your recovery
When you first contact OPHP, you will speak to their intake team who will provide you with information about the program and can also send you a list of general mental health and substance use resources.
If you are interested in participating in the program, the OPHP intake team will collect your information and provide you with the necessary paperwork to get started. You will then be connected to a case manager who will help guide the next steps.
No referral is needed. OPHP can be contacted directly by phone at 1-844-931-0007 or by email at [email protected]
Some of the reasons you might reach out to the OPHP include:
- You are struggling with your mental health or substance use.
- Your normal mental health supports are not as effective, or you need new supports.
- You need help navigating the mental health system.
- Your mental health or substance use is affecting your relationships, work or ability to engage in day-to-day activities.
You can always call 1-844-931-0007 to discuss whether OPHP might be right for you before committing to the program.
When pharmacists and pharmacy technicians are dealing with mental health and substance use challenges, including addiction, anxiety, depression or other conditions, the College’s goal is to support individuals to access the resources they need so that they can practice in a safe manner.
The College has an obligation to protect patients and ensure registrants are practicing safely, and, as part of that commitment, we want registrants to get the help they need to be healthy. It is in the public’s best interest that registrants are given an opportunity to access this support.
Opening & Operating a Community Pharmacy
Contact Pharmacy Applications & Renewals if you have questions or want more information about purchasing, relocating or opening a new pharmacy; changing the name of a Designated Manager; updating the list of pharmacists authorized to order/receive and sign for controlled substances; changing the pharmacy’s hours of operation; changing the pharmacy’s dispensing fees; completing the annual renewal; or other questions about opening or operating a pharmacy in Ontario.
Email: [email protected]
Phone: 416-962-4861 ext. 3600
Fax: 416-847-8399
No. Subscription to a Drug Information Service is optional.
At a minimum, every pharmacy should have at least one reference in each of the following areas:
- A Canadian Drug Reference / Compendium
- A Drug Interaction Publication
- A Drug Therapy Publication
- A Patient Counselling Guide
By removing the Board-approved list of specific references, pharmacy professionals gained the flexibility to select the resources in each area they deem most appropriate to support them in the delivery of patient care. Registrants are also encouraged to assess their practice and select additional references based on the pharmacy’s patient population and the professional services provided. References should be reviewed, evaluated and updated on a regular basis to ensure each remains relevant, current and suitable for its intended purpose: to allow registrants to meet the Standards of Practice.
When a pharmacy is accredited, the Point of Care sign, the Usual & Customary Fee and Notice to Patients signs will be provided by the College after completion of a satisfactory accreditation assessment and are to be posted as soon as possible after they are received.
Signage for time-delayed safes can be downloaded directly from the Time-Delayed Safes webpage.
Signage for Remote Dispensing Locations can be accessed via the Checklist for Opening a Remote Dispensing Location Staffed by a Pharmacy Technician.
To order replacements of any physical signage, please email: [email protected].
To change the pharmacy’s operating hours, the Designated Manager or Director Liaison of the pharmacy must submit a written request to the College (attn: Pharmacy Applications & Renewals) detailing the name, address and accreditation number of the pharmacy, the new hours of operation and the effective date of the change. Send all requests to [email protected].
To change the dispensing fee for a pharmacy, the Designated Manager or Director Liaison of the pharmacy must submit a written request to the College (attn: Pharmacy Applications & Renewals) detailing the name, address and accreditation number of the pharmacy, the current dispensing fee, the new dispensing fee and the effective date of the change. Send all requests to [email protected].
To change the trading name of a pharmacy, the Director Liaison of the pharmacy must submit a written request to the College (attn: Pharmacy Applications & Renewals) detailing the current trading name, address and accreditation number of the pharmacy, the new name by which the pharmacy will be known to the public and include a copy of the prescription label for the pharmacy with the new trading name. Send all requests to [email protected]
Banner: Banner groups are groups of retail pharmacies similar to franchise groups. They are principally marketing groups that allow for joint advertising and promotion. They are formed for the purpose of providing support to retail pharmacies, which are typically individually owned and not part of a parent company.
Franchise: A franchise is a method of distributing products or services involving a franchisor, who establishes the brand’s trademark or trade name and a business system. A franchisee pays a royalty and often an initial fee for the right to do business under the franchise name and system. Unlike a chain business, where a parent company owns all the business locations, a franchise is typically owned by separate individuals, who oversee running their own pharmacy.
The table below lists the known Banners and Franchises currently operating in Ontario.
| Banners in Ontario | Franchises in Ontario |
|---|---|
| Allied Pharmacists Inc. | Jean Coutu |
| Care & Cure | Hooper’s Pharmacy |
| IDA | Shoppers Drug Mart |
| Guardian | The Medicine Shoppe |
| OnPharm-United | |
| PharmaChoice | |
| Pharmasave | |
| Remedy’s Rx | |
| Rx HealthMed | |
| Total Health | |
| Whole Health |
If you do not see your Banner or Franchise listed above, check the list below for examples of corporations/organizations which are not considered a Banner or a Franchise.
Organizations not considered a Banner or Franchise
CATP (Canadian Addiction Treatment Pharmacy)
Costco
Drug Trading
Enterprise
Loblaw
McKesson
Metro
Rexall
Sobeys
United Pharmacy Group
Walmart
The College’s requirement is for the pharmacy to have a separate and distinct patient consultation area in the pharmacy offering ‘acoustical privacy’. This ensures patients can talk to the pharmacist in an area of the pharmacy to discuss their medications or health without being overheard by other customers.
It is up to the Designated Manager to determine what is appropriate for their space. Once Pharmacy Applications has accepted the completed application, a Community Operations Advisor is assigned to review your floor plan and follow up with you with any concerns.
All pharmacies, regardless of the nature of the business or services provided, must meet the standards for accreditation and operation, as defined in the Drug and Pharmacies Regulation Act, 1990 and as established by the College.
Please refer to Accreditation and Operation of a Pharmacy Guidance for more information and links to relevant resources.
A Certificate of Accreditation may only be issued to a “bricks and mortar” pharmacy at a specific municipal address. There is no separate class of accreditation for online or internet pharmacies.
All pharmacies must meet the standards for accreditation and operation, as defined in the Drug and Pharmacies Regulation Act, 1990 and as established by the College. Please refer to Accreditation and Operation of a Pharmacy Guidance for more information and links to the relevant resources.
An accredited pharmacy may conduct business over the internet according to the College’s Operating Internet Sites Policy. This policy requires the pharmacy’s website to have its accreditation (“license”) number and the name of the Designated Manager so it can be verified by the pubic on the College’s public register.
The distance between pharmacies is not mandated by the College nor a legislated requirement. You may wish to check with your municipality regarding by-laws or zoning restrictions that may apply to your proposed location.
Hours of operation are at the discretion of the Designated Manager and not mandated by the College nor a legislated requirement.
The number of hours a pharmacy is open should be consistent and communicated to the public, who should know when a pharmacist is available to dispense prescriptions or answer questions about medication.
Changes to a pharmacy’s hours should be emailed to [email protected].
Opioid Strategy for Pharmacy
Ontario is facing a serious public health and safety issue related to opioid substance use disorder. As the regulator of pharmacy in Ontario, putting patients first has been and will always be our number one goal. We recognize that no single initiative will “fix” Ontario’s opioid-related issues. The College is committed to aligning with national and provincial opioid-related goals.
We know that, as healthcare professionals, pharmacists and pharmacy technicians have an important role to play in responding to serious public health issues affecting our patients, communities and the province at large. The College believes that the profession of pharmacy can help with improving opioid-related care for patients, encouraging harm reduction, preventing overdose and addiction, and ensuring quality oversight of the provision of narcotics and controlled drugs to patients and proper destruction of any unused medications.
There are many complex health, social and system factors that have contributed to this current crisis. However, this is a public health crisis that requires action on all fronts.
As medication experts, pharmacists are in a unique role to support the appropriate use and access to narcotic and other controlled drugs. They are also in a position to engage in collaboration with other health care professionals to enhance patient safety. Additionally, pharmacy professionals are often the most accessible healthcare providers for patients and so may have opportunities to develop and identify best practices and provide additional support and education around opioid issues.
Hospitals are encouraged to review the framework’s recommendations to look for opportunities to enhance their own management of Controlled Substances in high risk areas. There should be collaboration across the hospital to identify any gaps and implement any necessary changes to policies, procedures, reporting, or training.
Hospitals are encouraged to establish key performance indicators (KPIs) to evaluate their progress on improving the safety and security of Controlled Substances. Such indicators may include, but not be limited to, the number of Health Canada loss and theft reports, the number of confirmed diversions and the results of physical security and process audits on a regular basis to track the impact of their diversion prevention and mitigation strategies.
Pharmacists and pharmacy technicians working in hospitals are encouraged to become familiar with the recommendations, identify any gaps that might exist in their practice setting, and bring the framework to the attention of pharmacy and hospital leadership. It will be important to look for opportunities for collaboration with other healthcare professionals to identify where there is a role for pharmacy professionals to adjust, enhance or share practices around Controlled Substances
The members of the Partnered Table to Improve the Safety and Security of Controlled Substances in Hospital High Risk Areas, including the College, will work collaboratively to identify future opportunities to measure the impact of the recommendations from the framework on diversion within hospitals and to hear feedback on implementation.
College hospital operations advisors will be sharing and discussing the framework with hospital teams as part of their regular operational assessments in addition to activities and dialogue already underway during these visits regarding ensuring the security of Controlled Substances.
The sponsorship of the framework by the College is part of our Opioid Strategy. Therefore, the College will also focus on encouraging implementation of the recommendations within hospitals by working collaboratively with various health system stakeholders.
Various health system stakeholders were involved in the information gathering, drafting and review of the framework.
To ensure a wide regulatory lens was reflected in the framework, both the College of Physicians and Surgeons of Ontario and the College of Nurses of Ontario were given an opportunity to review the framework and provide feedback. They have also agreed to post the framework on their websites for use by their registrants.
PACE
To apply for PACE, you must have already met the education requirement and be registered as an intern or intern technician. As an intern/intern technician, you can (and are encouraged to) gain practice experience to prepare for the Jurisprudence, Ethics and Professionalism Exam, PACE and the PEBC Qualifying Exam (particularly the OSCE or OSPE). These three registration requirements can be done in any order.
Your intern/intern technician certificate of registration is valid for one year and must be valid until the final date of your PACE assessment.
The list of PACE practice sites is constantly changing as assessors are selected and released daily. You should check the list of available practice sites frequently as this will change. Please note that site releases only happen during the College’s hours of operation (Monday to Friday, 8:30 a.m. to 5 p.m., excluding statutory holidays).
Rather than just looking in your hometown, you should also look for sites in surrounding communities that are a reasonable commuting distance from your home. It may be necessary to travel or stay somewhere else temporarily.
We are actively recruiting assessors through all College communication channels and recommendations from the OCP practice advisors. We review assessor applications continuously and hold online assessor training workshops throughout the year.
Yes, you may select a site that is part of your employer’s organization provided you have not trained, worked or volunteered at that site, and you have no prior relationship with the staff. For example, your PACE practice site could be part of the same hospital network or the same pharmacy chain or banner of your current workplace. You must declare your relationship to the practice site on the online application. A College registration advisor will review the circumstances to determine if you and your proposed assessor will be allowed to complete PACE together. Please review the bias or conflict of interest in PACE document to understand how your assessor’s ability to objectively assess you as a candidate may be affected.
If you are currently employed as an intern technician at an in-patient hospital pharmacy and are looking for a hospital-based PACE site, please e-mail [email protected] with your name and OCP number before applying for PACE. An OCP staff member will confirm your place of practice and provide you with next steps.
When you select a PACE site and proposed start date, your request is sent to the assessor. They will determine their availability and may need to adjust the start date. You should wait until your PACE has been confirmed by the College before you make any unalterable commitments about time off work or accommodations.
Most PACE candidates are unpaid. The College does not require assessors or their pharmacy practice site to pay PACE candidates because they are attempting to meet a practice-based registration requirement. Some organizations may choose to pay candidates who are employed by them.
If you need to or decide to discontinue PACE at any time after your start date has been confirmed and before the end of your assessment, you must immediately inform the College at [email protected] and provide your reason(s) for not being able to complete PACE.
Yes, assessment accommodations for applicants with a documented disability are provided as outlined in the Application Process section of the PACE policy. The online PACE application asks if you require assessment accommodations. Requests for accommodation must be received by the College with no less than six weeks’ notice of your preferred PACE start date.
No, you may not take a vacation break during or between your orientation and assessment phases. You should not plan any time off until at least four weeks after your PACE start date to ensure sufficient time to complete your entire assessment. If an unexpected illness or emergency arises during your PACE, you must contact the College as soon as possible by e-mailing [email protected].
If a situation develops during the assessment period that you believe may have affected your performance, you should immediately inform your assessor and/or a College registration advisor by emailing [email protected]. You should also review the PACE Administration Appeals policy.
You are responsible for finding your own pharmacy professional coach. You may want to prepare a resume and cover letter that states you are searching for a coach to help you prepare for your PACE. When you meet a potential coach, you can share your performance profile and (if available) your learning action plan, and discuss the areas for improvement that were identified by your PACE assessor.
Pharmacies that are looking for pharmacy professionals may be interested in helping you if you are close to becoming fully registered. Check job postings and professional networks for pharmacist and pharmacy technician employment opportunities.
The coach you choose should be someone you believe genuinely wants to help you succeed in preparing for your next PACE attempt. You could ask your PACE assessor to be your coach, but they are not required to do so.
You should consider someone who meets the following characteristics:
- Provides pharmaceutical care to patients on a regular basis (i.e., identifying drug therapy problems, creating therapeutic plans, monitoring and following-up with patients)
- Is proficient in their ability to provide feedback and guidance
- Is in good standing with OCP with no terms, conditions or limitations on their certificate of registration (see OCP’s Public Register for this information)
- Demonstrates professionalism
- Models effective communication with patients, colleagues, and other health care professionals
- Is aware of and up to date on developments in practice
- Supports and understands the PACE model
- Promotes patient-centred care
- Has teaching or preceptor experience (optional)
Your coach’s practice site should:
- Promote patient-centred care;
- Provide sufficient practice opportunities to meet the candidates learning action plan goals and address their competency gaps (e.g., suitably high prescription count, variety of patients);
- Be a positive environment with sufficient staffing conducive to teaching and learning; and
- Have passed its recent pharmacy assessment and is entitled to operate in Ontario (see OCP’s Public Register).
You are required to report your PACE development practice site in your OCP account as you should be performing the authorized acts of the profession to prepare for your next PACE attempt.
If you fail PACE twice, you will have to:
- Make a request to a panel of the Registration Committee. The request should explain what the candidate has done to improve their practice competence since their first PACE attempt. The panel may order the candidate to complete additional education, examinations and/or training before they may apply to re-attempt PACE.
- Pay a reassessment fee (see line 21) to gain access to the PACE application.
Both these requirements will apply to the third and any subsequent PACE attempts.
Personal Professional Liability Insurance
You may wish to check with any pharmacy organization or association to which you belong or any insurance carriers who provide you with other insurance (e.g., home, automobile). The College does not endorse or recommend any particular insurance carrier.
Yes, you must continue to have PPLI coverage while you are a Part A registrant.
You also have the option to resign from the College Register and reinstate within three years from the date of your resignation. You will not be required to have PPLI once you resign but will need PPLI to be eligible to reinstate directly into Part A of the Register. See Resigning & Reinstating for more information.
Your PPLI must meet the OCP By-Law requirements. Before starting to complete your final application for registration as a pharmacist or pharmacy technician, please contact your insurance provider to ensure that your PPLI coverage as a pharmacist or pharmacy technician will meet the requirements.
Your employer may pay for or reimburse you for your PPLI premium. However, the insurance must be in your name and stay with you if you change employers.
Yes, you are required to have separate PPLI coverage. The PPLI policy must be issued in your name and provide you with mobility and coverage wherever you practice in Ontario.
Pharmacy Technicians
No. “Pharmacy Technician” (and its abbreviation “RPhT”) became restricted titles under section 10 of the Pharmacy Act in December 2010. All other titles – such as Certified Pharmacy Technician (in use prior to 2008) – must no longer be used. This does not prevent someone from indicating their past achievement of passing the College’s certification exam, such as on a résumé, however the title of “Pharmacy Technician” cannot be used in practice unless the individual is registered with the College.
The Pharmacy Act and Ontario Regulation 256/24 define Scope of Practice and authorized acts for all pharmacy professionals. The steps involved in conducting a BPMH, such as gathering patient records from various sources and identifying discrepancies, are not necessarily governed by legislation. However, the regulation does set out Terms, Conditions and Limitations on a pharmacy technician’s certificate of registration, and pharmacy technicians cannot provide information or education relating to drug use which requires therapeutic knowledge, clinical analysis or assessment (s18.4).
Pharmacy technicians responsible for performing a BPMH must have the appropriate knowledge, skills and training to meet the Standards of Practice for Pharmacy Technicians outlined in #9-#12. When collaborating to provide patient care, technicians are expected to document their decisions and actions in the patient’s health record, including the relevant health and drug information they’ve obtained and their interpretation of this information.
The Pharmacy Manager/Administrator should establish policies and procedures for conducting medication reconciliations, outlining the respective roles for pharmacy technicians and pharmacists. OCP resources to consult include the Hospital Pharmacy Assessment Criteria, Standards of Operation, and the Pharmacy Technician Practice Assessment Criteria. External resources are also available from organizations such as the Canadian Society of Hospital Pharmacists (CSHP), ISMP Canada, the Canadian Patient Safety Institute (CPSI), etc. Collaborating with peers at other hospitals to share operational advice and best practices is also encouraged.
The College’s Opioid Policy expects pharmacists providing Opioid Agonist Treatment (OAT) to practice in accordance with CAMH’s Opioid Agonist Maintenance Treatment: A Pharmacist’s Guide to Methadone and Buprenorphine for Opioid Use Disorder and the Standards of Practice.
When dispensing methadone, the pharmacist is responsible for the therapeutic check of the prescription, assessing the patient, and witnessing the administration and ingestion of observed doses. These functions cannot be delegated to a pharmacy technician by the pharmacist.
Part A pharmacy technicians have the independent authority to check the accuracy of the technical components of methadone prescriptions. Pharmacy technicians may also be responsible for other tasks in the workflow that fall within their competencies such as checking the prescription for technical accuracy, dose preparation, assisting with documentation and inventory management, etc.
Additional resource
For example, can they check and sign for blister packs independently, or does that still require a pharmacist to cosign the hard copies? Also, are they able to process, fill and sign for a repeat prescription independently or does it have to be cosigned by a pharmacist as well? My assumption is that they would be able to do these things since the original therapeutic check was already done by a pharmacist who checked and signed the original prescription and they would be just refilling it?
The legislation (O. Reg. 256/24) states “Every certificate of registration as a pharmacy technician is subject to the following terms, conditions and limitations: 1. The member shall only engage in the practice of pharmacy (…) when practising in a pharmacy to which the Drug and Pharmacies Regulation Act applies, other than a remote dispensing location, while under the direct supervision of a member holding a certificate of registration as a pharmacist…”
A pharmacy technician can check any/all prescriptions for technical accuracy and completeness. Every prescription – new and refill, regardless of how they are packaged – must have both the technical and therapeutic check completed prior to release to the patient. Please refer to the integrating pharmacy technicians into the dispensing workflow webpage for more details; note that the term “co-signing” is not used, as each registrant is performing an independent “check” for different purposes.
A suitable method for documenting both “checks” for a given prescription may be signatures (or some other identifying mechanism) from both registrants on every hardcopy. However, other methods may be suitable to document the respective actions and associated responsibilities in the dispensing process. As software, workflow, personnel, etc. can vary from pharmacy to pharmacy, the information presented on our website is not meant to be entirely prescriptive for how a pharmacy achieves the desired end result, which is to ensure the patient receives a prescription that is both technically accurate and therapeutically appropriate.
Ultimately, registrants remain accountable for their respective roles in dispensing a prescription, and documentation on the patient record should reliably demonstrate that every prescription – new and refill – has been reviewed for both technical and therapeutic aspects before it is dispensed. It should be readily retrievable, auditable, and be unambiguous. When and how these functions occur, and what form they take (i.e., operational procedures) are at the discretion of the pharmacist and technician in consultation with the Designated Manager.
Additional Resource
Police Background Checks
The cost for an E-PIC from Sterling Talent Solutions is $30 plus HST (subject to change). The costs to obtain a police background check from your local police station may vary, but are typically anywhere from $20- $50.
If you obtain your police background check through Sterling Talent Solutions, your background check will be available within 24 hours. If you obtain your police background check through your local police station, processing times may vary, but are typically up to two weeks.
If the police background check was issued within six months of the final processing of your application for the certificate of registration, you may submit it provided it is authenticated with a police services stamp if submitted as a paper document, or is available for the College to retrieve from a police background check website online. If it does not meet these requirements you must complete another one.
Enhanced Police Information Checks (E-PICs) completed online through Sterling Talent Solutions will be retrieved by the College directly through their secure system. If you are submitting a paper document obtained through your local police station, you may send the original to us via mail or in person provided it is authenticated with a police services stamp. The original paper documents will not be returned to you.
Practice Assessment
You will need to demonstrate your ability to meet the Standards of Practice during your practice assessment. You should discuss with your supervisor(s) how and when you can gain experience and become confident in the scopes of practice you plan to use as a Part A registrant upon successful completion of the practice assessment.
For community pharmacists: You are required to gather examples of pharmacist prescribing (this could include adaptations, pharmacist authorized renewals and minor ailment prescribing), medication reviews, and drug therapy problems you identified and managed ahead of the assessment. New and refill prescription examples will be gathered and reviewed during the practice assessment; you do not need to prepare these examples prior to the assessment.
For pharmacy technicians and hospital pharmacists: Refer to the hospital pharmacist practice assessment and pharmacy technician practice assessment pages for documentation examples. Prior to the assessment, your practice advisor will discuss with you on a call what examples to have prepared in advance and how your processes will be reviewed.
You should have an example of each scope of practice that you are planning to implement as a Part A registrant.
Participation in the Quality Assurance Program is a requirement under the Regulated Health Professions Act. Failure to participate in the practice assessment when selected will result in a referral to the Quality Assurance Committee. The Committee has the authority to direct actions that can significantly impact your ability to practice, including transferring your certificate of registration from Part A to Part B of the Register.
The deadline to submit your documents is 2 weeks prior to your assessment date; however, you may submit your documents earlier if you choose.
If you are unsuccessful in your post-remedial practice assessment, you will be given the opportunity to spend time with a different quality assurance (QA) coach.
Following the session with the QA coach, you will be reassessed onsite by a different practice advisor. If this reassessment identifies significant areas that require improvement, you will be required to undergo a QA assessment again with a different College-appointed peer QA assessor.
The QA Committee will review the results of your first and second QA assessment and recommend required remediation based on your needs.
When you are notified that you must undergo a QA assessment, you should review the reports from your first and second practice assessments, and the learnings from your QA coach to apply the recommendations into your practice. You should also seek other relevant resources to improve your practice.
If you do not meet the standards required on your first practice assessment, you will be given the opportunity to spend time with a quality assurance (QA) coach. This coach is not a College staff member, but rather a peer who can provide support specifically in areas where there is room for improvement. This half-day interactive session is designed to enhance your practice and the care you provide to patients. Following the session with the QA coach, you will be reassessed onsite by a different College practice advisor.
If your second practice assessment identifies significant areas that require improvement, you will be required to undergo a QA assessment by a College-appointed peer QA assessor. You will also be required to complete the Knowledge Assessment.
The QA Committee will review the results of your QA assessment and Knowledge Assessment and advise on next steps based on your needs, which may include remediation or self-directed learning. If remediation is required, you must complete a post-remedial practice assessment onsite within one year of completing remediation.
To complete your practice assessment, you must be physically present at your Designated Practice Assessment (DPA) site in Ontario.
If you exclusively provide patient care outside of Ontario (e.g., in another Canadian jurisdiction or the U.S.) and do not have a DPA in Ontario, you do not meet the requirements of a Part A registrant. Please reach out to [email protected] to discuss your options of resigning or transferring to part B of the register.
During a practice assessment, practice advisors use the practice assessment criteria to evaluate your practice. Throughout the assessment, the practice advisor will:
- provide feedback outlining areas where you are doing well and meeting standards
- identify areas where there is an opportunity for improvement
- offer support through coaching and conversation
- probe the thinking behind certain actions and activities
- indicate where to access helpful resources
After your assessment, you will receive feedback in a report available to you in your online OCP account. Results are typically available within 48 hours. The report is focused on opportunities for learning and resources to help practice.
The results of a practice assessment are confidential and are not shared with employers, pharmacy owners or colleagues. The results are only provided to the Quality Assurance (QA) Committee according to the process described below.
You will typically be notified that you have been selected for a practice assessment two to three months in advance. To prepare, you should review the practice assessment criteria specific to your place of practice. The “Guidance” section explains how the specific performance indicators will apply in practice, including examples.
You are strongly encouraged to self-assess your current practice before your College practice assessment. The practice assessment is not a test of your clinical or therapeutic knowledge. Continuing to practice to the full scope of the profession is your best preparation.
You will be requested to submit specific documentation at least two weeks before your scheduled practice assessment.
For specific criteria and documentation requirements, please select your place of practice:
The practice assessment may or may not fall on the same day as a scheduled shift. If your practice assessment date falls on a scheduled shift, it is strongly recommended that you have an extra pharmacist/pharmacy technician available to provide patient care and oversee the dispensary operations while the assessment is taking place.
If you are not scheduled to work at the pharmacy on the practice assessment date, it is your responsibility to coordinate with your manager to ensure you have a confidential area to complete your assessment. As the practice assessment is part of the College’s Quality Assurance Program, it does not have to take place during your working hours.
It is your responsibility to inform your manager or lead of your practice assessment.
The initial practice assessment is conducted virtually through Microsoft Teams while you are at your place of practice. It will take between two and three hours.
You must have an area within the pharmacy that is acoustically private and confidential. You are expected to be alone in this area for the duration of the assessment. If this is not possible due to lack of space in the pharmacy, you must use earbuds or headphones.
Reassessments and the practice assessment to move from Part B to Part A of the Register are conducted in person.
No. Practice advisors will only assess the processes that a pharmacy technician is responsible for in their place of practice. For example, if a pharmacy technician does not perform sterile compounding, they would not be asked about it.
Your practice advisor will ensure the discussion addresses your practice processes in providing care to your patients. They will use the four domains of the practice assessment criteria that are relevant to your place of practice.
Approved Deferral Reasons
A deferral may be requested only for the following reasons:
- medical reason (with documented evidence)
- maternity/paternity leave (expected return date must be provided)
- pre-booked travel (with documented evidence and expected return date)
If repeated deferral requests are received, your case will be referred to a Quality Assurance Advisor for review.
Unforeseen Circumstances
Certain unforeseen circumstances, such as urgent medical or family issues or accidents may be considered on a case-by-case basis for a deferral.
Late Cancellations
A late cancellation is a cancellation made less than six weeks before the scheduled assessment date.
These cancellations are NOT due to an approved deferral reason, or an unforeseen circumstance listed above. For your first late cancellation or missed assessment, you will be rescheduled at the discretion of the practice advisor. Any subsequent late cancellations or missed assessments will incur a fee (see the Schedule for Fees for the current fee amounts).
We take patient privacy very seriously at the College. All document submissions containing personal health information (patient name, etc.) are done via Microsoft’s SharePoint platform. SharePoint allows files to be securely collected from external parties without granting access to the folder’s contents.
Redaction of patient information should be done according to your organization’s policies. Practice advisors do not need access to patient identities to perform the assessment. However, it is important for you to know the identity of your patients to provide any additional information during the assessment. If redacted copies are sent to OCP, you should keep a record of the patient’s name for your own use.
If you are using a personal device for any part of the submission, you should be redacting your documents. If redacting, do so fully and on a copy, not the original documents. Be vigilant for the presence of patient identifiers; these may appear in water marks found on prescriptions. Failure to fully redact, when necessary, may result in a privacy breach.
You may need to forward the SharePoint link to your work email for ease of sending documentation after redaction.
You must demonstrate how you meet the Standards of Practice during your assessment. The primary way that you do this is by sharing and discussing examples with your practice advisor that are representative of your everyday practice. If you cannot provide and discuss appropriate examples during the assessment, you risk falling below in a key performance indicator, which could require the completion of additional quality assurance activities.
Submitting your documentation at least two weeks ahead of time means you will be better prepared and also supports a more efficient assessment. If your documentation is not submitted ahead of time, does not meet the requirements for your role/practice setting or is not sufficient to assess your practice, the practice advisor will expect you to screen share practice examples during the assessment. Therefore, you must ensure you have authorization to share this information, including access to patient profiles at the pharmacy.
The College is committed to providing the highest level of security, controls and integrity to support the safe transfer of the information you provide.
Microsoft SharePoint’s file request feature allows files to be securely collected from external parties without granting access to the folder’s contents. The designated link provided to you does not allow any user to view, edit or download any existing files. Access is provided solely for the purpose of file uploads.
More information on the security standards and data encryption used in SharePoint is available in this Microsoft article.
All documents submitted to the practice advisor for the purpose of the practice assessment will be securely destroyed 15 days after the assessment documentation has been uploaded to OCP’s Customer Relationship Management (CRM) system according to OCP’s Records Retention Schedule and Privacy Management practices.
No. Documentation should be submitted exactly as it looked when you completed it. The practice assessment is meant to be a review of your day-to day practice and your examples submitted should reflect this.
Preferred Provider Networks
No. The College cannot make laws or rules for the practices of employers, insurance companies or pharmaceutical manufacturers. As PPNs are established by employers who negotiate and purchase group benefit plans from third parties on behalf of their patients, we do not have any current ways to limit their use.
However, we are exploring a number of options to address our concerns and will focus on taking direct action where we have the authority to do so.
We also believe that business models that restrict patient choice are another example of business pressures being applied to pharmacy decisions that interfere with pharmacy professionals’ ability to deliver the kind of care they want to provide to their patients.
A business model or agreement that limits patients’ choice of pharmacists and pharmacies can interfere with continuity of care and timely access to pharmacy services. Patients can be deprived of pharmacy care that meets their diverse needs, whether related to language, cultural safety or anything else that might factor into their choice of a health care provider. Where patient care may be compromised, the College is concerned.
Here’s an example: A patient has been receiving regular medications from their chosen local pharmacy for five years. But because their employer-provided health insurance has entered an agreement with a specific chain of pharmacies only, they must go to a different pharmacy in the next town to receive specialty medication. Now the patient needs to choose between splitting their prescriptions at different pharmacies (which can increase the risk of errors), switching everything to an unknown pharmacy that they can’t easily access or staying with their trusted pharmacy and paying extensive drug costs out of pocket.
Process to Move from Part B to Part A
For the purpose of preparing for their chosen practice assessment, a Part B registrant (with written approval from the Registrar) may perform the listed controlled acts* in a pharmacy under the direct supervision of a Part A pharmacist, or a Part A pharmacy technician (for Part B pharmacy technicians only).
If the Part B registrant has chosen the PACE option for their practice assessment, they will be assessed on their ability to safely and independently practice with minimal guidance under their assessor’s supervision. If the Part B registrant has chosen the practice assessment by a College practice advisor option, they should demonstrate preparedness and provide patient care examples based on their experience performing the controlled acts. Therefore, it is crucial that a Part B registrant be given sufficient opportunities during their practice preparation period to perform the controlled acts under the direct supervision of their supervisor.
All other terms, conditions, and limitations on a Part B registrant’s certificate of registration continue to apply. For example, a Part B pharmacist cannot:
- Supervise a pharmacy or the part of the pharmacy where drugs are kept
- Supervise the practice of the profession by another person
- Be a Designated Manager (DM) of a pharmacy (as defined in the Drug and Pharmacies Regulation Act)
*Note: Controlled acts performed for the purposes of providing a publicly funded drug or service must be in accordance with Ministry of Health policies, which may limit participation to Part A registrants. Please refer to the Ontario Public Drug Programs Executive Officer Communications for the latest updates and program requirements. Additionally, while the Part B registrant may be able to perform a controlled act, for example prescribe for the purposes of adapting or extending a prescription, they may not be able to bill for this act under the Ontario Drug Benefit Program. In the case that these acts are billed under the supervisor’s name and OCP number, there should be clear documentation on who was involved in the provision of that service.
Once you have completed and submitted the application form to notify OCP that you wish to move to Part A, you may perform the controlled acts in a pharmacy under direct supervision (i.e., supervisor must be physically present) by the Part A registrant(s) listed on the form; Part A pharmacy technicians may only supervise Part B pharmacy technicians. Initially, you should observe the Part A registrant(s) in practice and then transition to providing patient care under supervision as appropriate based on your level of competence. Please see the Supervision of Pharmacy Personnel Policy for more information on the level of supervision required.
Any Part A pharmacist or a Part A pharmacy technician (for Part B pharmacy technicians only) may be a supervisor. If training and coaching pharmacy professionals is new to the supervisor, they are encouraged to complete modules 1, 3 and 6 of this preceptor training program and to review the Part B registrant’s desired scope of practice.
The Part B registrant must have consent from the Part A registrant to be their supervising Part A registrant and must notify the College in writing of the supervisor’s name and practice site and their anticipated start date of practice. A Part B registrant may have more than one supervisor; however, all supervisors must be listed on the application form.
The Part B registrant and the Part A supervisor will have shared accountability for the Part B registrant’s actions, including errors and how they learn from them.
Part B registrants who are practising in preparation for the practice assessment are required to hold personal professional liability insurance.
Direct supervision (i.e., the supervisor is physically present on the premises) is required when supervising a Part B registrant, in accordance with the Supervision of Pharmacy Personnel Policy. The Part B registrant should observe the Part A registrant in practice and transition to providing patient care under supervision as appropriate based on their level of competence. The degree of oversight of the Part B registrant’s practice may be adjusted based on their demonstration of competence. The Pharmacy Connection article, Six Things to Consider when Supervising Pharmacy Practice, is an additional helpful resource in determining the level of supervision needed. The level of supervision may change over the course of a supervisory relationship. For example, the Part A supervisor may allow the Part B registrant to work more autonomously as they progress, based on the supervisor’s judgement and observations of the Part B registrant’s competence.
Referral to Panel & Appeals
Your employment confirmation letters should include the start and end dates that you worked or volunteered at the company, the average number of hours per week you worked, your job description and specific duties, and how well you performed your work. Information that relates to the reason your application is being referred to a panel (e.g., additional education/training, fluency, character) should also be included. The letter should include the date it was written, and include the writer’s full name and contact information. If the person writing the letter is a registrant of the College, they should also include their OCP number.
No. When you submit an Application for a Certificate of Registration, you must pay an application fee. If you do not meet all the requirements for that certificate of registration or are asking for an exemption from a requirement, your Application will be referred to a panel of the Registration Committee. Once you are eligible for registration (regardless of the Panel’s decision), the fee you initially submitted will be applied to that specific application provided you register within one year from the initial application date.
Registration Fees and Timelines
You may submit your documents for evaluation by the PEBC before your arrival. The PEBC Pharmacist Evaluating Exam may be offered in London, England for an additional fee. Please refer to the PEBC website for more information.
You may submit your language proficiency test results from any testing institution that offers one of the tests accepted by the College. Please refer to the website for each testing agency to find out where their testing institutions outside of Canada are located.
The online Orientation to the Canadian Health Care System, Culture and Context course that is part of the bridging education for international pharmacy technician applicants may be completed before your arrival. The program recommends that you only take this course if you are within three to six months before arriving in Canada.
The self-study reading and activities for the online IPG Program may be completed while you are overseas. You must provide evidence of your Canadian status to register for the IPG Program. Please refer to the IPG Program website for more information.
The Registration Committee has authorized College staff to extend the validity of some requirements (e.g., language proficiency test scores). A panel of the Registration Committee may consider extending the validity of some requirements. Jurisprudence, Ethics and Professionalism exam results will not be extended. Please contact [email protected] for more information about expiry dates for your requirements.
Applications to the College are generally processed within 1-2 months (including ~6 weeks for applicants coming through Gateway). May take longer during peak times. The fees and timelines charts for each pathway to registration list the processing time for various applications.
Remote Dispensing
In addition to what is required on all prescription labels, the container in which a drug is dispensed from a remote dispensing location (RDL) must indicate both the name, address and telephone number of the accredited pharmacy as well as the address of the RDL including a toll-free number at which a patient may contact the accredited pharmacy. The label must include a unique identifier, attached to the prescription number that identifies that the drug was dispensed from the RDL vs. the accredited pharmacy.
Every remote dispensing location (RDL) where a pharmacist is not physically present must be equipped with a live, two-way audio-visual link that permits dialogue and communication between the patient and a pharmacist who is physically present in the accredited pharmacy. In the event of a disruption in this link, all dispensing at the RDL must cease immediately and cannot resume until the link is restored.
In the case of an automated pharmacy system (APS), the technology used to transmit the prescription must be approved by the College. Methods to ensure verification of physician signature and authenticity of prescriptions remain consistent with all pharmacy practices. For more information, please see the Opening a Remote Dispensing Location webpage.
- Every remote dispensing location (RDL) must be designed, constructed and maintained so as to prevent unauthorized access.
- Must have an alarm system that provides immediate notification to the Designated Manager (DM) or their delegate of any theft or attempted theft of drugs, tampering or attempted tampering of RDL or its equipment and alternation in refrigeration temperature outside of standards
- Once the alarm system has notified the DM all dispensing at the remote dispensing location will be stopped until the DM confirms that the remote dispensing location, including any automated pharmacy system, is fully secured and operational.
As well, if the RDL has an automated dispensing system, it must:
- Be locked at all times to prevent unauthorized access and be sufficiently affixed within the RDL so that it cannot be moved by unauthorized persons.
- Use technology, such as bar-coding or micro chips, that ensures that drugs are accurately loaded and verifies that the correct drugs are selected robotically during the dispensing process and correct labels affixed to vials.
- Employ College-approved technology for the creation of a digitally scanned image of a paper-based prescription
A remote dispensing location (RDL) must be located in a well-lit and well-ventilated area that is appropriate for the provision of health care services and accessible to the public only during the hours that a pharmacist is physically present in the accredited pharmacy that operates the RDL. There is no restriction regarding proximity to another pharmacy.
The same requirements that apply to any pharmacy must also apply to the remote dispensing location (RDL) which include a dispensary. The pharmacy must be at least 18.6 sq metres and dispensary 9.3 sq metres. A RDL in which all drugs are dispensed and distributed from an automated pharmacy system is not required to have a dispensary or meet the pharmacy size requirements.
Only the pharmacy whose certificate of accreditation permits the operation of the remote dispensing location can operate it at the specific location referred to in the certificate of accreditation. The operation cannot be contracted out to another pharmacy.
A pharmacy can operate multiple remote dispensing locations (RDL) but each RDL will have a specific accreditation number. Additional fees for each RDL application will be required and specific standards of accreditation must be met. As with any new store opening, an inspection will take place to ensure the standards are met prior to accreditation approval.
A holder of a certificate of accreditation in the community pharmacy class can apply for an amended certificate of accreditation that permits the operation of remote dispensing location(s). For more information, please see the Opening a Remote Dispensing Location webpage.
Drugs in Schedule I, II and III may be sold in a remote dispensing location. Schedule III drugs may be available either from an automated pharmacy system or from an area in the remote dispensing location to which the public does not have access. No narcotic and controlled drugs and targeted substances can be located at or available from the RDL.
Yes, the pharmacist must be physically present in the pharmacy that operates the remote dispensing location.
A “remote dispensing location” means a place where drugs are dispensed or sold by retail to the public under the supervision of a pharmacist who is not physically present.
Restocking of Drugs Used for Medical Assistance in Dying (MAiD) During COVID-19
It is the College’s expectation that the accredited hospital pharmacy ensures that the drug has been in the possession of a licensed healthcare professional at all times (from time of dispensing to time of return to the pharmacy). If the drug has left the possession of a licensed healthcare professional, even for a short period of time, the drug integrity cannot be verified and restocking is not permitted. The licensed healthcare professional who has been in possession of the drug should confirm that the drug has been stored in accordance with the manufacturer’s requirements (e.g. appropriate temperature, etc.).
The O. Reg 130/17 of the Pharmacy Act considers it “professional misconduct” to return to stock, resell or redispense a Schedule I drug that was previously sold or dispensed. Therefore, registrants must only engage in restocking for MAID drugs under the authority of the accredited hospital pharmacy where the pharmacy has established this practice as permitted through the DPRA, O. Reg. 264/16, s.32.
Only accredited hospital pharmacies are permitted to engage in the restocking of drugs in accordance with the stipulations identified in the policy. Due to provincial regulation, community pharmacies are currently not able to engage in the restocking of drugs. Currently, the Drug and Pharmacies Regulation Act, DPRA, O. Reg. 264/16, s.32 permits the restocking of drugs (subjection to the conditions in the regulation) in an accredited hospital pharmacy environment. The DPRA, O. Reg. 264/16, s.32 prohibits an accredited community pharmacy returning to stock, reselling or redispensing a Schedule I drug that was previously sold or dispensed.
Given this is a situation where expeditious advice is needed, and this policy is only intended to be in effect for the duration of the COVID-19 public health emergency in Ontario, the College presented this policy to the Board for temporary application during the COVID-19 public health emergency, and truncated the typical policy development process. Once the emergency order has been lifted in Ontario, the College will continue to monitor the status of the drug shortages and determine whether development of a permanent restocking policy is warranted. A permanent policy would follow the typical policy development process including a public consultation.
There is currently a shortage of drugs used in the Medical Assistance in Dying (MAiD) protocol due to the COVID-19 pandemic increasing demand for these drugs to treat patients on ventilators in hospital intensive care units, as well as an overall increase in demand for MAiD. Once the emergency order has been lifted in Ontario, this policy will be rescinded, and the College will continue to monitor the status of the drug shortages and determine whether development of a permanent restocking policy is warranted.
Scope of Practice
From a scope of practice perspective, a pharmacist may conduct a comprehensive medication review for a patient according to the Standards of Practice.
Establishing the MedsCheck program eligibility criteria for patients and pharmacists falls under the mandate of the Ministry of Health. Information related to billing, such as submitting a claim for reimbursement and the required paperwork, can be found in the materials available on the Ministry of Health website, such as the Professional Pharmacy Services Guidebook, and the Ontario Pharmacists Association’s MedsCheck Resources. Additional questions can be directed to the ODB HelpDesk or [email protected].
For example, the Ministry of Health may require the pharmacist to meet certain education requirements to be eligible to receive reimbursement for providing a Ministry of Health professional service program. As pharmacists are self-regulated professionals, the College cannot interpret the Guidebook on behalf of a registrant and/or determine whether the Ministry’s criteria have been met.
Yes. The Drug and Pharmacies Regulation Act (DPRA) s149 permits pharmacy students and pharmacy technician students under direct supervision of a Part A pharmacist who is physically present, to perform their respective therapeutic and/or technical checks to dispense prescriptions. Students should document their actions, reflecting which step(s) of the process they performed and are responsible for, on the dispensing record.
The College does not require a specific documentation method or format for “signing” a prescription by the student or the supervising pharmacist. This depends on the software, technology, and workflow in place, which differs between pharmacies. As with any activity performed by a student, there must be a way to readily identify the supervising pharmacist who is responsible and accountable for the student. The Designated Manager/pharmacy administrator should establish expectations and operational procedures on documentation to support students and supervising pharmacists. Refer to the Supervision of Pharmacy Personnel Policy for more information. Consideration should be given to situations outside of the College’s mandate, such as third-party billing and audits. Students should consult their educational institutions for guidance on documentation during experiential learning.
Yes. Federal regulations authorize pharmacy technicians to accept verbal prescriptions and to transfer prescriptions for drugs on the Prescription Drug List. These regulations define pharmacy technician as a person who is registered or otherwise entitled under the laws of a province to practise pharmacy and is practising in that province.
In Ontario, students enrolled in a pharmacy technician education program can practice pharmacy under the supervision of a Part A pharmacist without being registered because of an exception in the Regulated Health Professions Act. Since they meet the definition of pharmacy technician for the purposes of the regulations, they can accept verbal prescriptions and transfer prescriptions.
Pharmacy technicians, pharmacy technician students, and intern technicians cannot perform either of these activities for prescriptions for controlled substances (narcotics, controlled drugs, and benzodiazepines & other targeted substances).
For a summary of the legislation, refer to the Legal Scope of Practice & Authorized Acts chart.
Yes. Federal regulations authorize pharmacists to accept verbal prescriptions and to transfer prescriptions. These regulations define pharmacist as a person who is registered or otherwise entitled under the laws of a province to practise pharmacy and is practising in that province.
In Ontario, students enrolled in a pharmacy education program can practice pharmacy under the supervision of a Part A pharmacist without being registered because of an exception in the Regulated Health Professions Act. Since they meet the definition of pharmacist for the purposes of the regulations, they can accept verbal prescriptions and transfer prescriptions.
Verbal prescriptions can be accepted for drugs on the Prescription Drug List (“prescription drugs”) and certain controlled substances — specifically controlled drugs, verbal prescription narcotics, and benzodiazepines & other targeted substances.
For transfers, prescriptions for prescription drugs can be transferred by a pharmacy student to another pharmacy student or to any registrant of the College. However, prescriptions for benzodiazepines & other targeted substances must be transferred to another pharmacy student, intern or pharmacist. Importantly, any of the additional activities permitted by the Health Canada subsection 56(1) class exemption under the Controlled Drugs and Substances Act cannot be performed by pharmacy students or interns, because the exemption defines pharmacist as a person “who is entitled under the laws of a province or territory of Canada to practise as a pharmacist.”
For a summary of the legislation and exemption, refer to the Legal Scope of Practice & Authorized Acts chart.
Yes. Pharmacy professionals are authorized to accept verbal prescriptions under federal regulations. The regulations outline what practitioners can order verbally and the details which must be included on the written record of the transcribed prescription. However, the regulations do not address the workflow or operational aspects that might be encountered in practice.
Regardless of who performs the ‘task’ of phoning in the prescription, the receiving pharmacy professional is responsible for determining that it is valid and authentic. If there is uncertainty about whether a practitioner with prescribing authority has truly initiated and authorized the order (being communicated via an intermediary), the pharmacy professional should follow up with the prescriber directly. The decision as to what is sufficient to verify the legitimacy of the prescription, considering the circumstances at hand and the best interest of the patient, rests with the professional judgement of the registrant who should document their rationale.
Pharmacy professionals should be especially diligent when receiving verbal orders due to the increased potential for errors.[1] Prescribers should consult their regulatory body for guidance on issuing verbal prescriptions.
Yes. A pharmacy professional may decide to administer a substance that is not listed in Schedule 2 of O. Reg. 256/24 to a patient via the intranasal route, without needing delegation of authority (e.g., a medical directive). Refer to Appendix A of the Administering a Substance by Inhalation Guideline as it explains that “intranasal” administration of a substance is not a controlled act and therefore is not restricted to health professionals or require enabling regulations.
With inhalation, the drug is intended to be delivered to the epithelium lining the lower respiratory tract. With intranasal administration, the drug is intended to be delivered to the mucous membranes lining the nasal passages. If a pharmacy professional decides to administer a substance by the intranasal route, this should still be done in accordance with the Administering a Substance by Inhalation Guideline, Code of Ethics and Standards of Practice.
Yes. Administering a substance by injection is a “controlled act”, as defined in the Regulated Health Professions Act (RHPA), that may only be performed by an authorized health professional. There are, however, provisions in the RHPA that permit “a person” to perform a controlled act “in the course of rendering first aid or temporary assistance in an emergency” even if they are not an authorized health professional.
Also, the province’s Good Samaritan Act provides protection from liability for a pharmacy professional “who provides emergency health care services or first aid assistance to a person who is ill, injured or unconscious as a result of an accident or other emergency”.
Therefore, in the event of a medical emergency, a pharmacy professional may decide to administer a substance (that is not listed in Schedule 1 of O. Reg. 256/24) to a person, without delegation of authority (e.g., a medical directive), while waiting for emergency medical services. This should still be done in accordance with the Administering a Substance by Injection Guideline, the Code of Ethics and Standards of Practice.
Pharmacy professionals may dispense or compound drugs pursuant to a prescription issued by a veterinarian for an animal, provided they have the knowledge and skills to do so safely and in accordance with the Standards of Practice. The Drug and Pharmacies Regulation Act (which governs dispensing and compounding by pharmacy professionals) defines ‘prescription’ as “a direction from a prescriber for the dispensing of any drug or mixture of drugs for a designated person or animal.” Importantly, the patient is the animal, not the owner/client, and collaboration between veterinarians and pharmacists is essential for the safety of their animal patients when providing these services.
However, it is important to recognize that additional factors are necessary to consider since the use of a drug in humans cannot be equated to the use of the same drug in animals1. Differences in the anatomy and physiology of animals can result in differences in a drug’s pharmacokinetic and pharmacodynamic properties and species-specific toxicities. The provision of care to animals requires specialized training and knowledge, and pharmacists have a professional responsibility to acknowledge when they lack sufficient competency to provide this service.
Other professional pharmacy services, such as prescribing (I.e., initiating, adapting, and renewing prescriptions) or administering substances, are governed by the Pharmacy Act. Regulations under this act define ‘prescription’ as “a direction from a prescriber directing the dispensing of a drug or mixture of drugs for a specific patient” without including ‘animal’. It presents a significant risk to animals for a pharmacist to provide care beyond the dispensing or compounding of a veterinary prescription. As such, the regulations that include these services in a pharmacist’s scope of practice, such as the recent change permitting prescribing for minor ailments, apply only to human patients.
Reference
Billing a prescription to a third party payer does not have any bearing on the scope of practice of pharmacy defined by the Pharmacy Act and Ontario Regulation 256/24. Vaccines (listed in Schedule 3 of the regulation) may be administered under independent authority in accordance with the Administering a Substance by Injection Guideline. Vaccines listed in Schedule I of the NAPRA Drug Schedules require a valid prescription in order to be dispensed prior to administration.
To receive reimbursement for influenza, which is a Schedule II vaccine, a claim is submitted to ODB via the Health Network System. This requires the pharmacist to enter information similar to that of a prescription, with the pharmacist’s identification in the ‘prescriber’ field (refer to the Ministry of Health and Long- Term Care’s Executive Officer communications). As this is a billing mechanism (which also creates documentation for the patient) it should not be interpreted as prescribing the vaccine, as pharmacists do not have the independent authority to do so.
Sterile Compounding
The College collaborated with the North East LHIN ’s Hospital Pharmacy Peer Group to develop an integrated strategy for hospital pharmacy medication management services as a region. The North East LHIN Regional Pharmacy Strategy was published on July 2018 and provides a framework to guide LHINs and hospitals to work together to make regional decisions that will ensure hospitals, as a region, collectively provide medication management services according to standards. The strategy acknowledges the importance of broader medication management practices but focuses on sterile compounding and prioritizes patient safety through the critical elements, patient access to services and optimizing volume of service delivery as a region. LHINs and hospitals may choose to use this framework to inform a coordinated regional approach to capital funding requests that accurately reflect the needs of the region. If LHINs and hospitals determine that there is a need for capital investments after going through the process outlined in the strategy, a LHIN-coordinated submission to the Ministry of Health for funding is recommended.
(LHIN = Local Health Integration Network. As of April 2021, Ontario’s LHINs have transitioned to Ontario Health as Home and Community Care Support Services)
The College has oversight of hospital pharmacies and assesses them to ensure that a safe medication management system is in place. The Drug and Pharmacies Regulation Act, s.119, defines a hospital pharmacy as “the primary location or locations in the hospital where drugs are compounded, dispensed or supplied from for hospital patients by a hospital, together with any other location in the hospital where drugs are stored or supplied from.” Therefore, if the hospital has medications for injectable use, then the sterile requirements would apply. If a hospital is receiving any compounded preparations from other sites or external sources, they must ensure that all compounded preparations received meet NAPRA requirements and have been maintained at the proper temperature during transport.
After an assessment, the designated individual receives an email with detailed instructions on how to access the Quill software system to respond to the requested action plan. Each issue identified during the assessment will be presented as an ‘Infraction’.
The action plan submitted must address the issues identified in enough detail, clearly outlining the steps taken to mitigate risk, optimize patient outcomes and sufficiently rectify the infraction. The response to each item should be concise, specific, achievable, and realistic with a defined implementation timeline. If sufficient detail is not provided, the operation advisor will communicate back using Quill requesting further information.
The action plan is required to be submitted 30 days from the date of the assessment; however, an ongoing collaborative dialogue may occur as the operation advisor supports achieving full compliance. It is the responsibility of the pharmacy/organization to continue to review their active infractions and status on an ongoing basis until the issues are appropriately addressed.
Continuing education providers are listed on the College’s website as a courtesy; the list is not exhaustive and does not imply the program and its content have been reviewed or endorsed by the College. The Designated/pharmacy manager and/or sterile Compounding Supervisor should confirm that any training or certification program completed by compounding personnel meets the competencies needed for their specific practice.
Determining the specific details of policies and procedures is the responsibility of the sterile compounding supervisor and Designated/pharmacy manager. The College does not approve a pharmacy’s operational procedures and policies.
Registrants are encouraged to collaborate with other pharmacy professionals in their region to share best practices and operational advice. Other resources include the references provided in the Standards, other reputable/recognized organizations and professional associations. During an assessment, College operations advisors will identify and discuss any concerns. The College’s approach focuses on achieving intended outcomes using risk mitigation strategies.
A third party evaluator (TPE) must meet the criteria set out in section 5.1.2.4 of the NAPRA Model Standards for Pharmacy Compounding Sterile Preparations.
The College does not evaluate or approve pharmacists or pharmacy technicians who wish to take on this role. It is the responsibility of the sterile compounding supervisor and Designated/pharmacy manager to do their due diligence and ensure the TPE meets the NAPRA Standards. To effectively “fulfill the mandate” and achieve the intended outcome of the standards, the TPE should possess knowledge and skills which correspond with the nature of the compounding practices being evaluated (e.g., experience with high-risk levels of contamination, handling of volatile drugs, with any specialized equipment or technology used, etc.).
Supporting Documentation for Registration
Your privacy is important to us. We are committed to protecting all personal information. This commitment to safeguarding your privacy is reflected in College’s Privacy Policy.
The College recognizes there may be some individuals who are unable to obtain the documentation required to complete their registration application and has procedures in place to handle these situations. For documents also required by the Pharmacy Examining Board of Canada (PEBC), the College will accept the verification process used by the PEBC for individuals in this situation.
Please contact [email protected] for more information about how to proceed with your application.
If you are required to create your OCP account directly with the College (i.e., are not required to enroll in the Pharmacists’ Gateway Canada), you may pay the initial application fee and begin submitting your supporting documentation up to one year before you expect to successfully complete your pharmacy education or to register in Ontario from another Canadian jurisdiction.
Time-Delayed Safes
All community pharmacies in Ontario will be using time-delayed safes. In addition, signs will be displayed at each public entrance to the pharmacy and in the dispensary (where patients drop off and pick up prescriptions). These signs indicate to the public that time-delayed safes are in use.
Thieves rely on getting in and out of the pharmacy quickly and knowing that narcotics are secured in time-delayed safes is a strong deterrent. The purpose of time-delayed safes and prominent signage indicating a time-delayed safe is in use, is to deter thieves from entering the pharmacy at all. Other jurisdictions that have implemented similar mandates saw an immediate decrease in pharmacy robberies, and the goal is to achieve a similar outcome in Ontario.
A time-delayed safe has an electronic timer that prevents access until a pre-set period of time has elapsed after the correct combination has been entered. The province-wide use of time-delayed safes has proven to be an effective robbery deterrent in other jurisdictions such as British Columbia and Alberta.
Although it may take a few extra minutes when picking up prescriptions, patients still have full access to the medications they require from their pharmacy. The introduction of time-delayed safes is another tool to support the security of medications and to help ensure medications are dispensed to patients only.
To protect patients and pharmacy teams, it is important to take preventative measures in the pharmacy space. Creating safer and more secure pharmacies benefits patients and pharmacy team members by reducing the risk of physical and emotional harm, and ensures safe access to care for patients. A safer environment also helps pharmacy professionals ensure the security of prescription drug supply in their communities.
All narcotics must be secured within the community pharmacy’s time-delayed safe.
Narcotics are any product or preparation which contains a drug named in the Schedule to the Narcotic Control Regulations.
If your pharmacy already has a safe without a time-delay mechanism, it may be possible to have a retrofit mechanism installed.
Costs for time-delayed safes vary depending on the size and provider. If your pharmacy already has a safe without a time-delay mechanism, it may be possible to have a retrofit mechanism installed at a lesser cost.
The College recognizes that this implementation requirement may represent an additional cost to the pharmacy. Purchasing a time-delayed safe is an investment in the safety of pharmacy team members and the public, and the security of drugs. There are many costs associated with having a pharmacy robbed, including time off for affected team members, counselling, replacing stolen stock and repairing any damage that occurred during a robbery.
No – all community pharmacies in Ontario are required to install a time-delayed safe as a way to protect patients and pharmacy teams. This decision was approved by the Board at its March 21, 2023 meeting.
Because the College does not have specialized classes of accreditation for community pharmacies based on business models, if your pharmacy is accredited as a community pharmacy by the College you must meet all the requirements of a community pharmacy. This includes installing a time-delayed safe.
The time-delayed safe expectation is outlined in Time-Delayed Safes Policy and the Standards of Operation for Pharmacies.
Because the potential to carry narcotics is always present as pharmacies meet the evolving medication needs of their patients, community pharmacies that currently do not have narcotics on site are still required to install a time-delayed safe.
A unified, Ontario-wide implementation approach is expected to have the impact of deterring pharmacy robberies in all community pharmacies across the province and will help protect patients and pharmacy staff.
The College takes compliance with mandatory requirements for the use of time-delayed safes for securing narcotics, and associated OCP-approved signage very seriously.
During operational assessments of community pharmacies, College staff will verify that the pharmacy has and is properly using a time-delayed safe. In addition, we are conducting random unannounced onsite visits to confirm community pharmacies are following these requirements.
Designated Managers at pharmacies that are not in compliance with time-delayed safes and signage requirements and policies may be subject to investigation. In addition, any follow up audits at the pharmacy to confirm compliance will incur a Pharmacy Re-Inspection Fee (see the Schedule of Fees for the current fee amounts).
Upcoming Changes to the AIMS Program
While updates to standards or policies require public consultation, decisions about the administration of College programs do not. Our Board’s decision is aligned with other provincial regulators that require pharmacies to choose their own platform and pay the associated costs. Where our approach differs is that OCP will cover the cost of submitting data to the NIDR to support a strong culture of medication and patient safety.
Yes. Pharmacies are still expected to meet the requirements of the AIMS Program during the 2026 transition of the program, including recording, documenting, analyzing, and sharing learnings about the incident.
If a pharmacy has not yet made a decision on which medication incident reporting platform they will use, then it must record medication incidents locally until such time that it has begun to use a chosen platform that meets program requirements. A template form will be made available on the OCP website as an option for pharmacies to record medication incidents.
Pharmacies that have decided to choose a new platform should review medication incident reporting solutions that meet the College’s criteria and sign up with the platform of their choice by January 1, 2027.
To fulfill the requirements of the AIMS Program, pharmacies must have access to an incident management platform that supports continuous quality improvement. It must meet all of the requirements set out by the College here.
All registered pharmacy staff require unique logins at their primary place of practice. Occasional staff, such as relief pharmacists, would not be required to have individual logins.
The safety self-assessment is an informative quality improvement tool that helps a pharmacy track their efforts to enhance patient safety over time. It can be used proactively to identify areas of potential risk, enabling pharmacy teams to plan improvement activities effectively and demonstrate system improvements. Requiring its completion every two years supports consistency and makes it easier for pharmacies to manage and meet deadlines. It also improves a pharmacy’s ability to maintain a safety culture by benchmarking data and tracking trends that can help identify and prevent medication incidents.
The changes – which include completing the safety self-assessment every two years and holding quarterly CQI meetings – align with national best practices and NAPRA model standards and are common in other provinces. They are intended to strengthen patient safety and support the development of a strong safety culture. Individual pharmacies still have the flexibility to determine how best to incorporate CQI program requirements into their workflows.
The information presented to the Board is available in recent Board materials packages. The most recent discussions about AIMS took place in June, September and December 2025. The College will continue to share updates and resources with registrants on an ongoing basis using all its regular communication channels.
A 60-day open consultation was held to gather feedback on the proposed changes to the AIMS supplemental Standard of Practice prior to approval at the December 2025 Board meeting. The Board decision to allow pharmacies autonomy to select their own medication incident reporting platform (provided it meets requirements and contributes to NIDR) as well as assigning responsibility for costs did not require consultation; however, information on this decision was included in the consultation materials posted.
All data collected from Pharmapod remains valuable and will continue to be part of our broader medication safety efforts. We have preserved historical data at an aggregate level and provided guidance on how the data could be backed up at individual pharmacy sites prior to December 31, 2025.
Sharing data at the national level helps to identify widespread risks and trends, enabling more effective coordinated interventions. It also supports consistency in safety practices, policy development, and shared learning across jurisdictions, ultimately improving patient safety across the country.
The AIMS Program launched in 2019, and the NAPRA Model Standards of Practice for CQI and MIR were published in 2021 as other provincial regulators were considering or taking steps to implement similar programs in their jurisdictions. An evaluation of the AIMS Program in 2024 created an opportunity to propose several changes, including alignment with the NAPRA standards to reinforce AIMS principles. To date, four other provincial Colleges have either adopted or adapted NAPRA standards.
In June, the Board made the decision to allow pharmacies autonomy to select their own medication incident reporting platform (provided it meets OCP’s criteria and contributes to NIDR). This decision was informed by the AIMS Program review which found low engagement with the program. Allowing pharmacies to choose a platform that best meets their unique workflows is intended to increase engagement with this important medication safety program. Ontario’s approach aligns with other provincial pharmacy regulators where pharmacies choose their own platform and pay the associated costs. Where it differs is that OCP will cover the cost of submitting the data to the NIDR to support a strong culture of medication and patient safety.
Allowing choice ensures the safety platform can integrate more smoothly with existing operations, reducing barriers to use by pharmacy staff. Giving pharmacies flexibility to select their own platform that meets their unique needs and workflows will ultimately improve participation and engagement.
Engagement with the AIMS Program is important because it enhances patient safety and promotes a culture of safety and continuous quality improvement in community pharmacies. Medication errors are preventable, and active participation in the program helps pharmacies identify system gaps and implement targeted improvements to prevent recurrences.
Aggregate data recorded on the platform helps identify common medication errors, contributing factors, and high-risk medications. The more pharmacy professionals engage with the program, the better the data that can be shared back to pharmacies – leading to greater opportunities to improve patient safety and reduce risk of harm.
In 2024, an evaluation found that engagement with the AIMS Program has remained consistently low, partly due to limited access to reporting by registered pharmacy staff. The evaluation also found that infrequent completion of the pharmacy safety self-assessment and an undefined frequency of continuous quality improvement (CQI) meetings have hindered the full integration of the AIMS Program in pharmacy practice. This level of engagement is not adequate for supporting the long-term sustainability and effectiveness of the College’s mandatory medication safety program.
The changes are informed by national best practices and alignment with NAPRA’s Model Standards of Practice for Continuous Quality Improvement (CQI) and Medication Incident Reporting (MIR) by Pharmacy Professionals, and are intended to improve engagement with the AIMS Program, strengthen patient safety, and support the development of a strong safety culture in community pharmacies.
The evolution of the AIMS Program, along with the addition and evolution of medication safety programs in other provinces, demonstrates the value placed on tracking and analyzing medication safety data in the public interest. It emphasizes the important role of pharmacies, regulators and other groups such as ISMP in collecting and sharing data across the country as a way to identify and prevent potential medication incidents from happening.